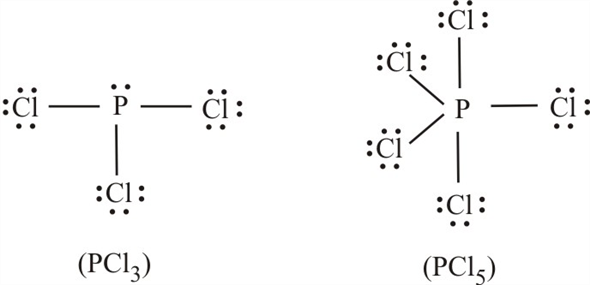
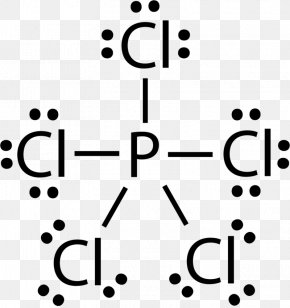
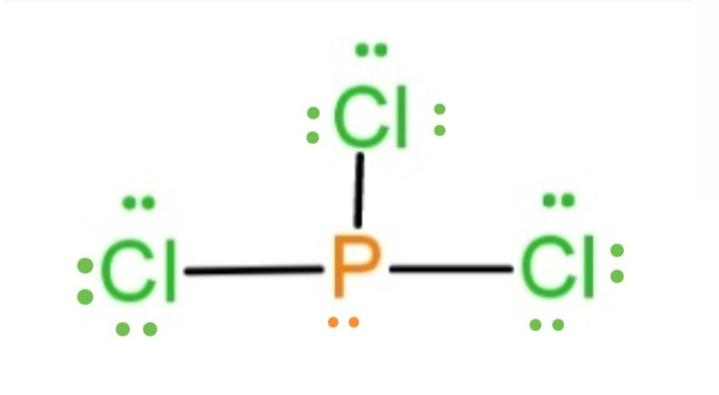
41 lewis dot diagram for pcl3
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
We can understand this concept with the help of either the concept of bonding or by using a simple formula. First, let’s understand the bonding part. We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms.
There are three chlorine atoms so draw 3 single bonds to p and write a cl at the end of each bond. Since they are in the same group on the ...

Lewis dot diagram for pcl3
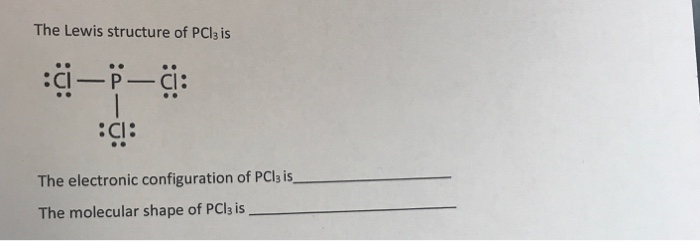
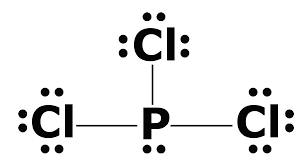
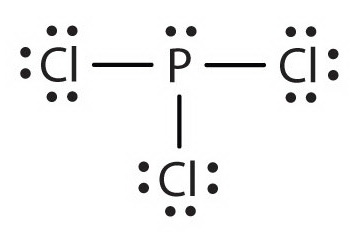
October 24, 2017 - Answer: P has 5 valence electrons in s2p1p1p1 configuration so the three unpaired electrons would bond with three Cl atoms to form a sp3 tetrahedral orbital hybridization. With only three Cl atoms around the P the molecule would be triangular pyramidal in shape and would be polar.
PCl3 Lewis Structure||Lewis Structure of PCl3 (Phosphorus Trichloride)||Draw Lewis Structure for PCl3#PClLewisStructureThis video will help you to draw the L...
The previous video, we saw some steps for drawing dot structures. In this video we’re going to use the same steps to draw a few…
Lewis dot diagram for pcl3.
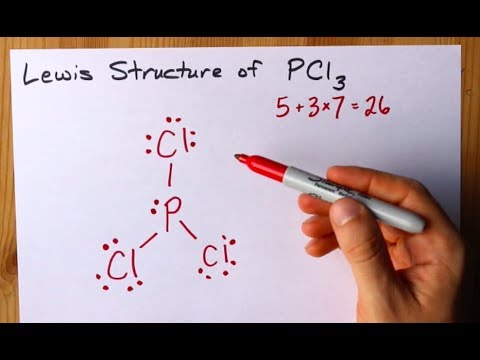
June 1, 2016 - Answer: Count total valence electrons. Chlorine 3x7=21; Phosphorus 1+5=5; 21+5=26. This tells us that these are all the electrons we have available to use in our structure. Next we write down P as the central atom and connect a single bond to each Cl. Each bond counts as two electrons so now we h...
In PCl3 lewis structure, each chlorine atom is joint with center phosphorus atom. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl3.
Check me out: http://www.chemistnate.com
Transcript: Hi, this is Dr. B. Let's do the Lewis structure for PCl3. Phosphorus, on the periodic table, is in group 5, it has 5 valence electrons. Chlorine, group 7, but we have three of those so we have 5 plus 7 (times 3 is 21) is 26 valence electrons. We'll put the Phosphorus in the center ...
March 11, 2020 - A. The Lewis Structure of PCl3. “PCl3 Lewis Structure” is published by Kamal Bisht.
July 23, 2021 - The hybridization of PCl3 can be determined once we know the Lewis dot structure of this molecule. Here three Chlorine atoms are bonded with Phosphorus atom, which means that there formation of hybrid orbitals that accommodate these shared electrons. Phosphorus’s electronic configuration ...
April 12, 2017 - May 13, 2016 - A step-by-step explanation of how to draw the PCl3 Lewis Dot Structure (Phosphorus Trichloride).For the PCl3 structure use the periodic table to find the tot...
The molecular geometry and polarity of phosphorus trichloride, PCl3 using VSEPR rules.
December 3, 2016 - Answer: A quick Google search will help you find it. If you want to know how this is found, check How to Draw A Lewis Structure In case you were wondering, the molecular geometry is trigonal pyramid.
Answer to Draw the Lewis structure of PCl3 on paper. (a) What is the total number of valence electron pairs in this molecule? (b) ...
August 31, 2020 - The molecular geometry of PCl3 is trigonal pyramidal and its VSEPR notation is AX3E. The “A” represents the central atom (the phosphorus), each X represents a chlorine atom, and the E represents the lone pair. You can watch me draw the Lewis Dot Diagram for PCl3 here:
April 4, 2016 - There are 3xx7+5=26 valence electrons to distribute, i.e. 13 " electron pairs". Around EACH bound Cl atom there are 3 lone pairs; there are 3xxP-Cl bonds; the thirteenth lone pair resides on phosphorus: :P(-Cl)_3. Since there are 4 electron pairs around phosphorus, the geometry is based upon ...
A. The Lewis Structure of PCl3 PCl3 Lewis Structure the compound has P and Cl Atoms. These Atoms Belong to the exceptions in the octet rule. These Atoms can exceed 8 surroundings electrons in the structure. Thus, we can't calculate the number of bonding and Non-bonding electrons. For this type of
I quickly take you through how to draw the Lewis Structure of PCl3, phosphorous trichloride. I also go over hybridization, shape and bond angle.

Warmup fill out the table below. make sure to draw the lewis structure in pencil! try your best! molecule so3 pcl3 o3 sicl4 beh2 total valence electrons.




















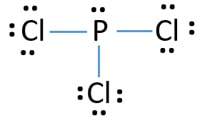



0 Response to "41 lewis dot diagram for pcl3"
Post a Comment