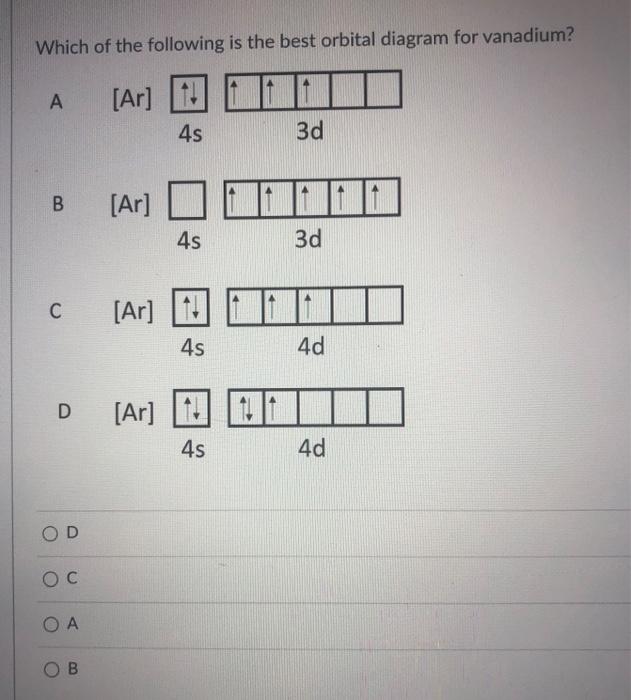
42 orbital diagram for vanadium
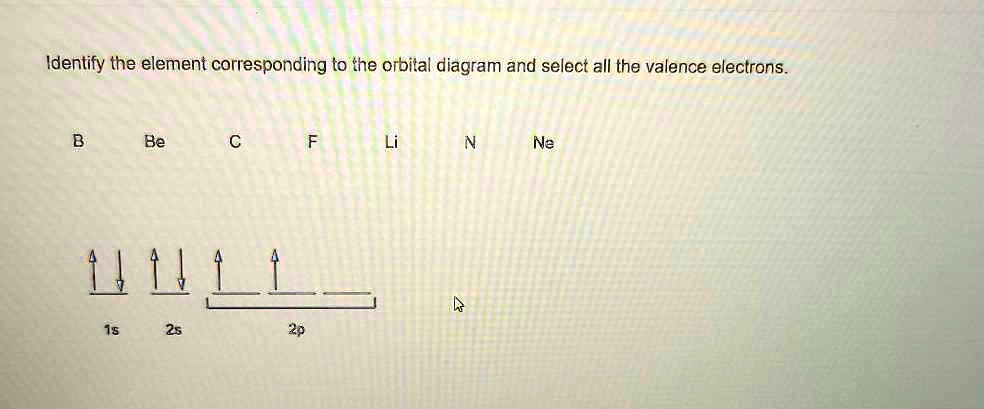
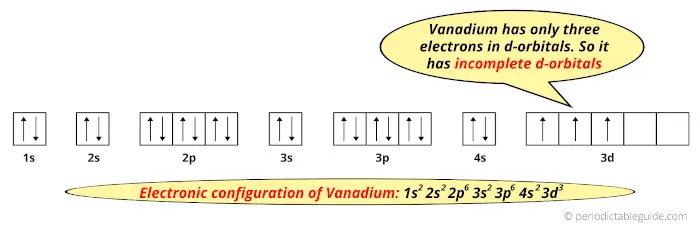
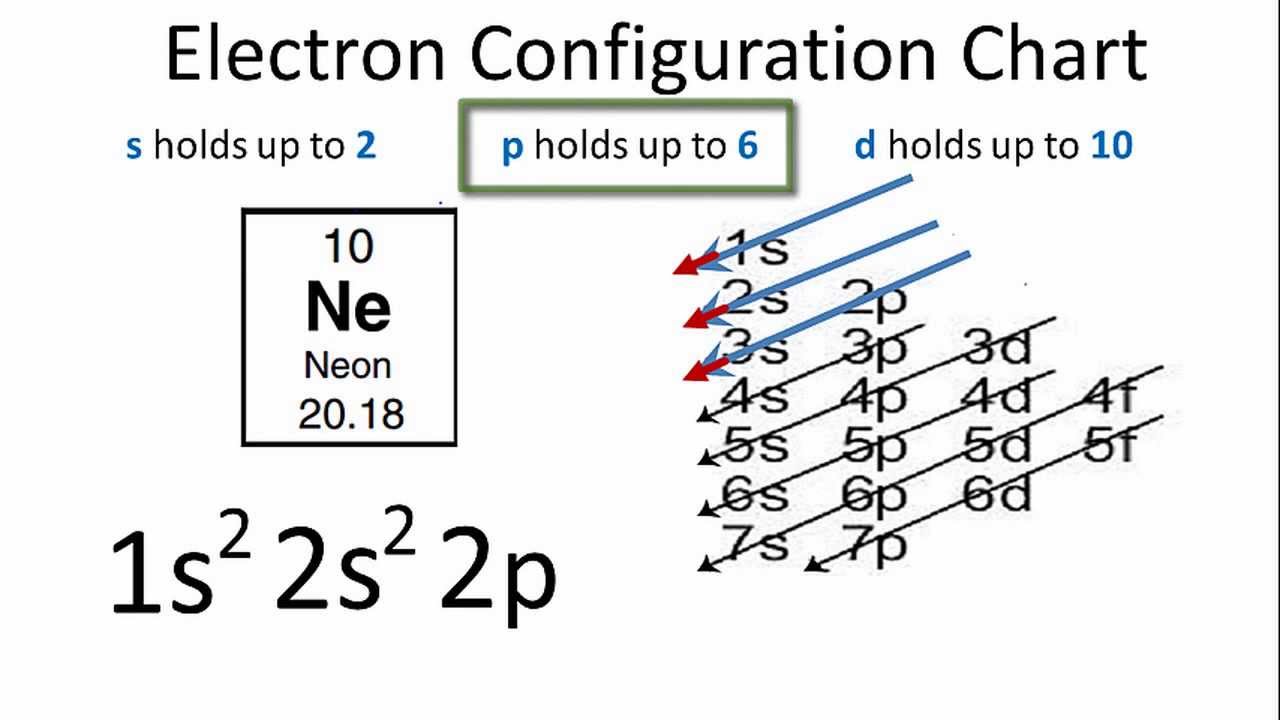
18.02.2021 · Orbital Diagram For Vanadium (V) | Vanadium Electron Configuration February 18, 2021 by Sneha Leave a Comment Vanadium Electron Configuration : When it comes to electronic configuration, it is one of the major topics in chemistry as we have mentioned before in our article. An orbital diagram, like those shown above, is a visual way to reconstruct the electron configuration by showing each of the separate orbitals and the spins on the electrons. This is done by first determining the subshell (s,p,d, or f) then drawing in each electron according to the stated rules above.
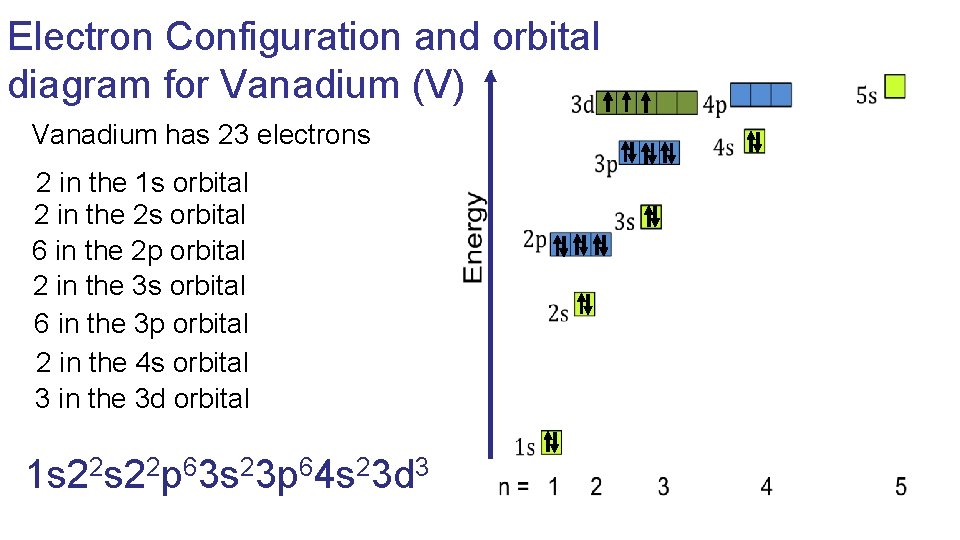
Nov 01, 2021 · Orbital diagram of Vanadium (V) 24: Orbital diagram of Chromium (Cr) 25: Orbital diagram of Manganese (Mn) 26: Orbital diagram of Iron (Fe) 27: Orbital diagram of ...

Orbital diagram for vanadium
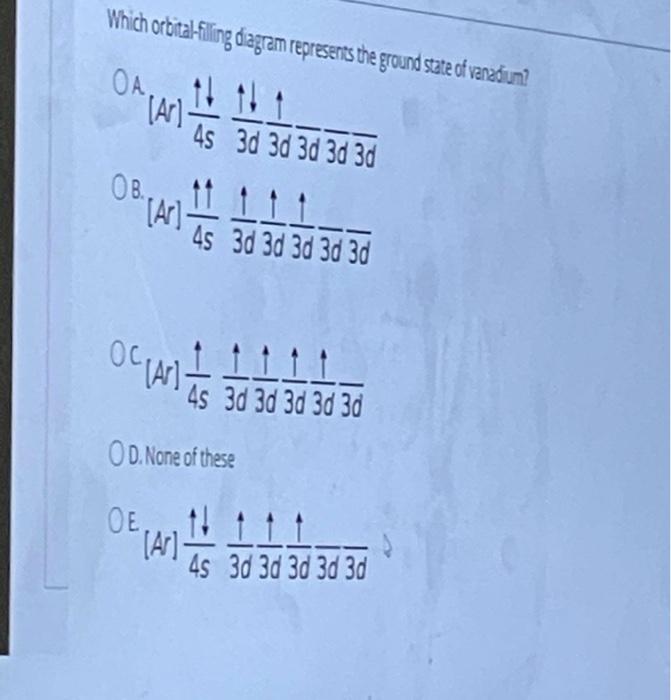
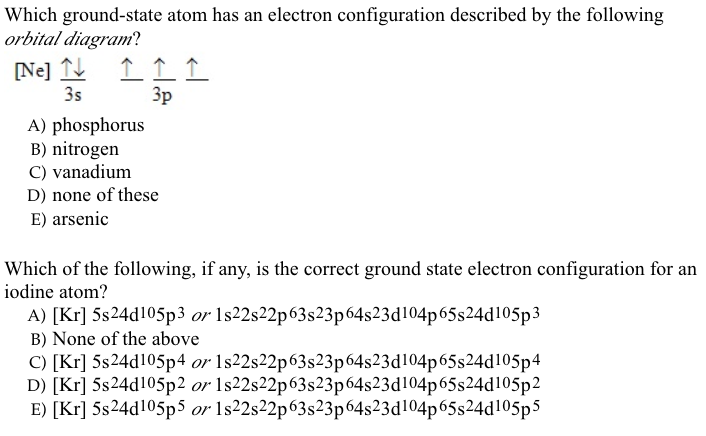
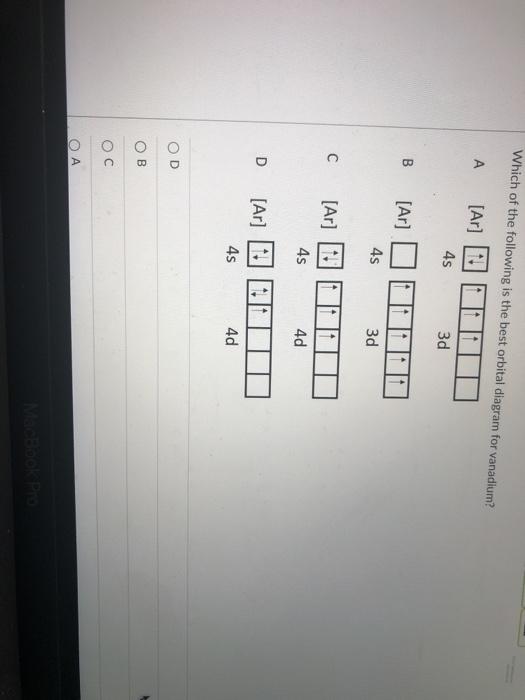
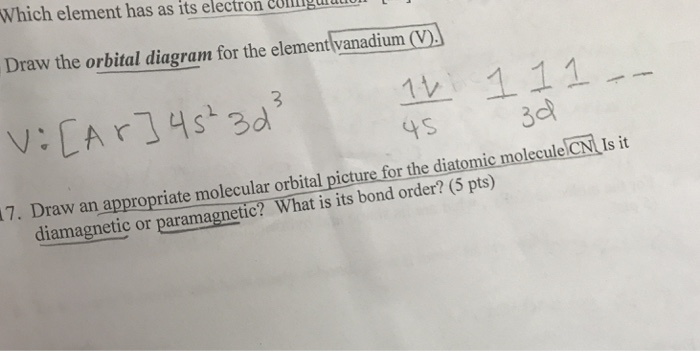
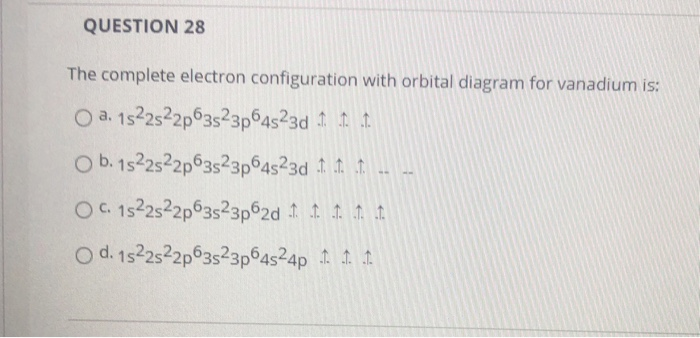
The aufbau diagram can be used to write correct ground-state electron configurations for all elements up to and including Vanadium, atomic number 23. The electron configurations for certain transition metals, like chromium and copper, do not follow the aufbau diagram due to increased stability of half-filled and filled sets of s and d orbitals. Feb 20, 2021 · Carbon Electron Configuration: If you guys have come across our recent article then it would be easy for you all to understand the concept.But if you are new here and looking for the information related to the carbon element and its electronic configuration, then today we will help you with some of the things and if you will be here till the last line surely you will go with some knowledge ... Identify the orbital (4 leaf clover) A) px orbital B) dxy orbital C) py orbital D) dyz orbital E) f orbital. A) F . Of the following, which atom has the smallest atomic radius? A) F B) Li C) Cl D) Na. C) 1s^22s^22p^63s^23p^64s^23d^5. Give the complete electronic configuration for Mn. A) 1s^22s^22p^63s^23p^64s^24p^5 B) 1s^22s^22p^63s^23p^64s^13d^6 C) …
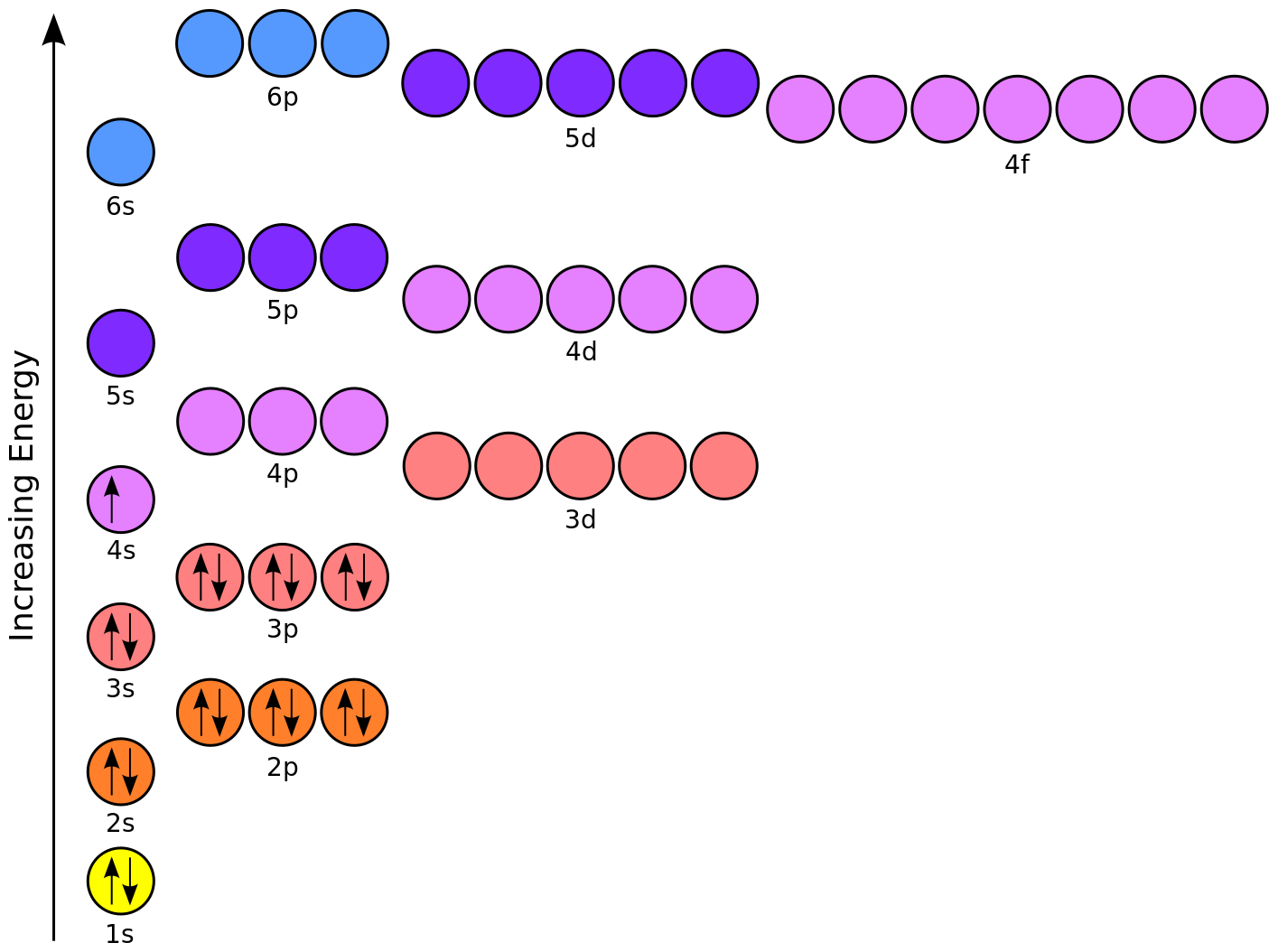
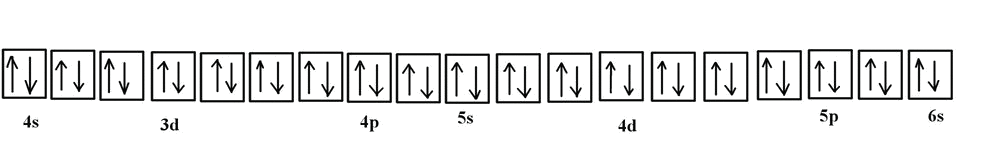
Orbital diagram for vanadium. For example, in the MO diagram provided for the [Ti(H 2 O) 6] 3+ the ns orbital – which is placed above (n − 1)d in the representation of atomic orbitals (AOs) – is used in a linear combination with the ligand orbitals, forming a very stable bonding orbital with significant ligand character as well as an unoccupied high energy antibonding orbital which is not shown. Electron configuration was first conceived under the Bohr model of the atom, and it is still common to speak of shells and subshells despite the advances in understanding of the quantum-mechanical nature of electrons.. An electron shell is the set of allowed states that share the same principal quantum number, n (the number before the letter in the orbital label), that electrons may occupy. 1. Orbital Filling Diagram 02 Ex. 2, Electron Configuration 02 Ex. (gives the most information) Is (quicker to draw than orbital filling diagrams) Dot Pb 3. Electron Dot shows only the valence (outer energy level) electrons Oxygen atom Ex. 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following ... Jul 06, 2019 · 04 Duramax Ob2 Wiring Diagram; Bodine Nsh-33r Wiring Diagram; Kubota Bx2200 Wiring Diagram; 1974 Yamaha Dt175-a Color Wiring Diagram; 50 Amp Square D Gfci Breaker Wiring Diagram; Venn Diagram Of Transcription And Translation; Vanadium Orbital Diagram; 92 Cadillac 4.9 Liter Wiring Diagram Inside Distributor; Craftsman Bench Grinder Wiring ...
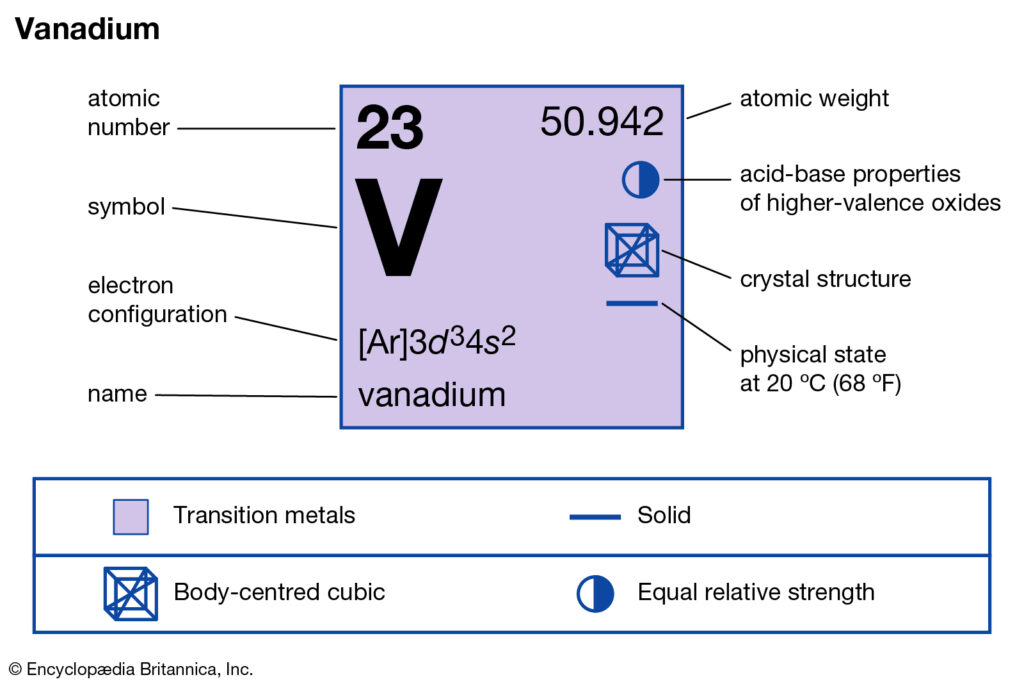
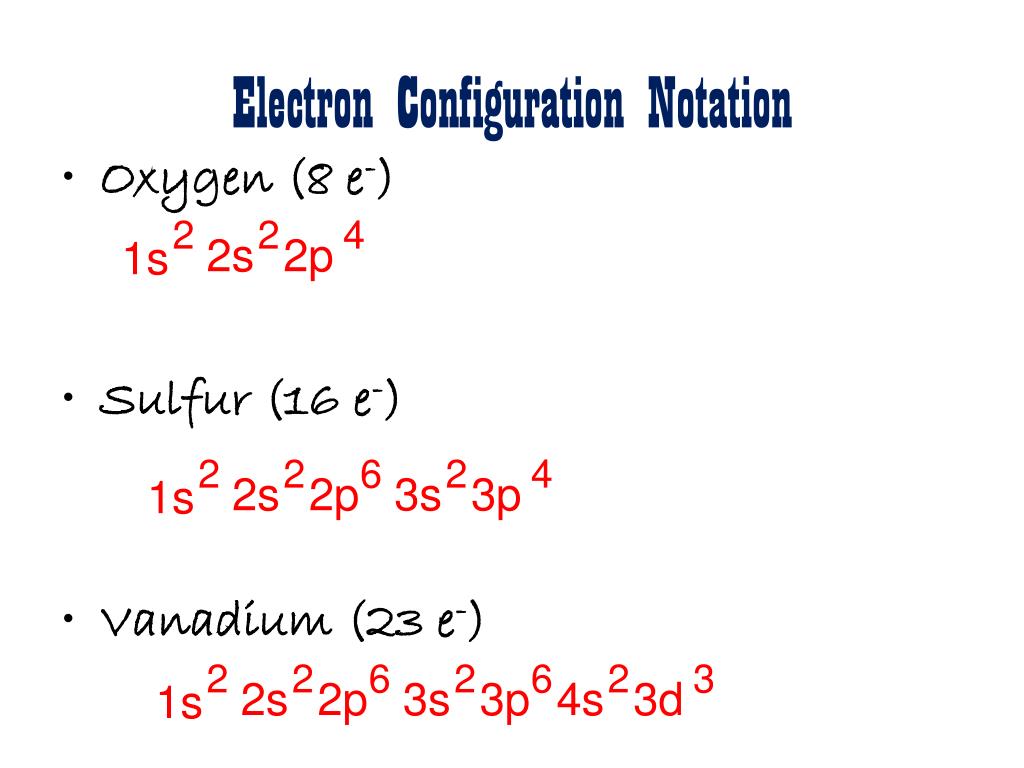
Vanadium Oxide – V 2 O 5; Molybdenum Disulfide – MoS 2; Copper (I) Oxide – Cu 2 O; BISCO Bismuth Strontium Calcium Copper Oxide – BSCCO; HgO-Mercury(II) oxide; Hexatantalate [Ta 6 O 19] 2-f-block Elements. Lanthanum(III) chloride – LaCl 3; Cerium Tetrafluoride – CeF 4; Gadolinium Orthoferrite – GdFeO 3; Garnet – Ca 3 Al 2 Si 3 O 12 – Grossular; Uranium (V) Chloride – U 2 Cl ... B) f orbital Basically, when l = 0 the is one orbital and it is called an s-orbital. It can hold 2 electrons total. When l = 1, the orbitals are called p-orbitals and there are 3 of them. Each of the individual p-orbitals can hold 2 electrons each. This gives us a total of 6 electrons that can go into the 3 p-orbitals. The electronic configuration of vanadium also includes 3 electrons in 3d orbitals electron configuration (shells): 2,8,11,2 . ... Work through the steps to draw the orbital diagram for O then o 2-(a) Draw orbital box diagram for O: 1s 2 2s 2 2p 4 (i) Apply Hund's Rule : Maximise unpaired electrons in the orbitals of a subshell before pairing up electrons 4 electrons in 2p subshell which is ... Identify the orbital (4 leaf clover) A) px orbital B) dxy orbital C) py orbital D) dyz orbital E) f orbital. A) F . Of the following, which atom has the smallest atomic radius? A) F B) Li C) Cl D) Na. C) 1s^22s^22p^63s^23p^64s^23d^5. Give the complete electronic configuration for Mn. A) 1s^22s^22p^63s^23p^64s^24p^5 B) 1s^22s^22p^63s^23p^64s^13d^6 C) …
Feb 20, 2021 · Carbon Electron Configuration: If you guys have come across our recent article then it would be easy for you all to understand the concept.But if you are new here and looking for the information related to the carbon element and its electronic configuration, then today we will help you with some of the things and if you will be here till the last line surely you will go with some knowledge ... The aufbau diagram can be used to write correct ground-state electron configurations for all elements up to and including Vanadium, atomic number 23. The electron configurations for certain transition metals, like chromium and copper, do not follow the aufbau diagram due to increased stability of half-filled and filled sets of s and d orbitals.

























0 Response to "42 orbital diagram for vanadium"
Post a Comment