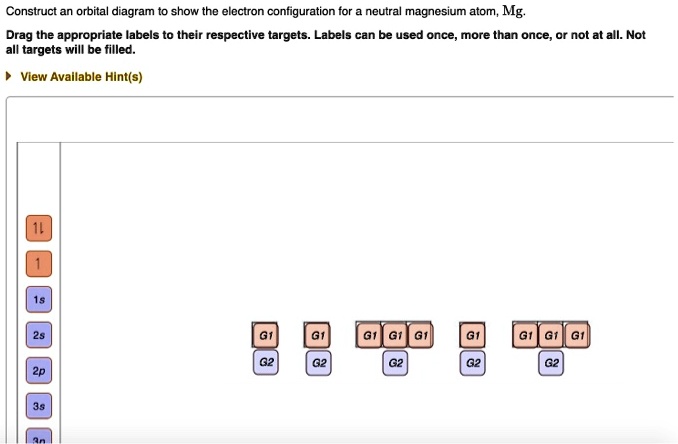
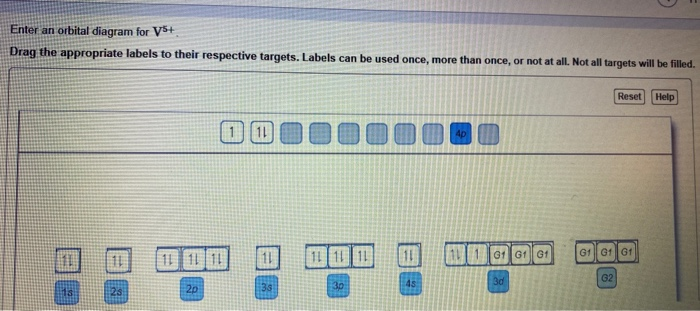
45 v5+ orbital diagram
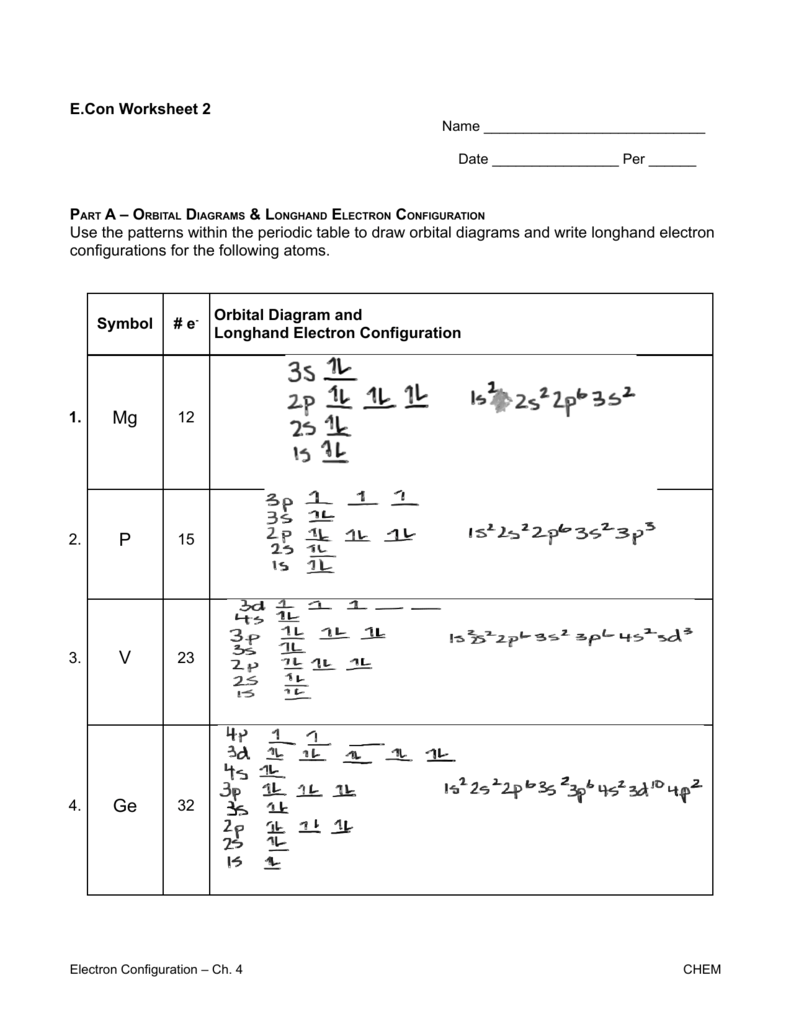
Write orbital diagrams for each ion and determine if the ion is diamagnetic or paramagnetic. a. V5+ b. Cr3+ c. Ni2+ d. Fe3+ Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V5+,Cr3+,Ni2+,Fe3+ Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high.

V5+ orbital diagram
Chemistry Q&A Library Write orbital diagram for V5+ Write orbital diagram for V5+ close. Start your trial now! First week only $4.99! arrow_forward. Question. Write orbital diagram for V 5+ check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here. For example, if the value of ‘n’ is equal to 3, the possible values of ‘l’, which range from zero to (3-1), are 0, 1, and 2. The names of these atomic orbitals will be 3s (for n=3 and l=0), 3p (for n=3 and l=1), and 3d (for n=3 and l=2). It can also be noted that it is not possible for the 3f orbital to exist because that would require ... To write the configuration for the Tin (Sn) and the Tin ions, first we need to write the electron configuration for just Tin (Sn). We first need to find the...
V5+ orbital diagram. V5+ Orbital Diagram. Since the 4s orbital is higher in energy, its electrons will be removed first. Not that it matters here, though, because exactly 5 electrons are. can be accommodated in the metal d orbitals. • d0 ions – Ti4+, Zr4+, V5+, Ta5+, Cr6+, Mo6+, etc. • d1 ions . σ-ML4 Tetrahedral MO Diagram e. In picture 1 we show the molecular orbital structure of F2. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. The dashed lines show the remaining p orbitals which do not take part in the bonding. σ z y x σ* x y z Construct the molecular orbital diagram for ... What element is represented by this orbital diagram? Pauli Exclusion Principle. 2 electrons in the same orbital must have opposite spins. Hund's Rule. Electrons don't pair up in orbitals of equal energy until they have to, and all electrons in singly occupied orbitals have the same spin. Orbital Diagram. Orbital diagrams are ways to assign electrons in an atom or ion. Each atomic orbital is represented by a line or a box and electrons in the orbitals are represented by half arrows.
Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at. Ni2+ Draw the d-orbital splitting diagrams for the octahedral complex ions of each of the following. a. Zn2+ b. Co2+ (high and low spin) c. Ti3+ the FT ligand. Just like there are 5 valence electrons for the element Vanadium. Similarly, every element will have its own valence electrons and many more. You can refer our article to those users or your friends who are looking for the information related to the Vanadium Electron Configuration of valence electrons as the good thing about our article is that it is available free of cost and no charges are ... To write the configuration for the Vanadium and the Vanadium ion, first we need to write the electron configuration for just Vanadium (V). We first need to ... This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
After the 4s is full we put the remaining six electrons in the 3d orbital and end with 3d6. Therefore the Iron electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Note that when writing the electron configuration for an atom like Fe, the 3d is usually written before the 4s. Both of the configurations have the correct numbers of ... The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of .. Rb+, Se2−. The first orbital (an s orbital) can contain only two electrons.. Rubidium. Answer to Write orbital diagrams for each of these ions. V5+,Cr3+,Ni2+,Fe3+ Determine if the ion is diamagnetic or paramagnetic. V 86%(14). Write orbital diagrams for each of these ions. A. V^5+ B. Cr^3+ C. Ni^2+ D. Fe^3+ E. Determine if the following ions are diamagnetic or paramagnetic. V5+ orbital diagram keyword after analyzing the system ... Write orbital diagrams for each of these ions. V5+ Cr3+ Ni2+ Fe3+ Determine if the ion is diamagnetic or paramagnetic. [Ne] 3s^2 3p^6 [Ar] 4s^0 3d^3 [Ar] 4s^0 3d^8 [Ar] 4s^0 3d^5 diamagnetic: V5+ paramagnetic: Cr3+, Ni2+, Fe+. Choose the larger atom from each of the following pairs. Al or In
To write the configuration for the Titanium ions, first we need to write the electron configuration for just Titanium (Ti). We first need to find the number...
Example of following the Aufbau principle, Pauli principle, and Hund's rule to construct an orbital diagram for a vanadium (Z=23) atom.
To write the configuration for the Tin (Sn) and the Tin ions, first we need to write the electron configuration for just Tin (Sn). We first need to find the...
For example, if the value of ‘n’ is equal to 3, the possible values of ‘l’, which range from zero to (3-1), are 0, 1, and 2. The names of these atomic orbitals will be 3s (for n=3 and l=0), 3p (for n=3 and l=1), and 3d (for n=3 and l=2). It can also be noted that it is not possible for the 3f orbital to exist because that would require ...
Chemistry Q&A Library Write orbital diagram for V5+ Write orbital diagram for V5+ close. Start your trial now! First week only $4.99! arrow_forward. Question. Write orbital diagram for V 5+ check_circle Expert Answer. Want to see the step-by-step answer? See Answer. Check out a sample Q&A here.

















.png)












0 Response to "45 v5+ orbital diagram"
Post a Comment