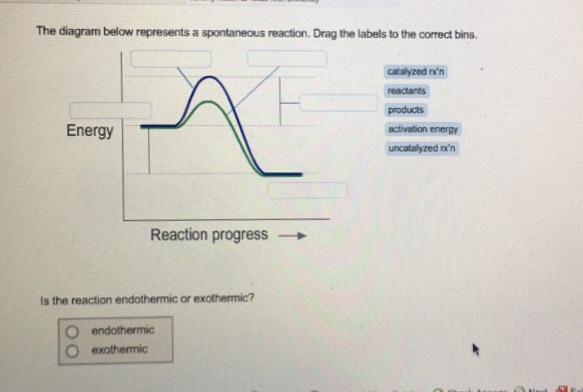
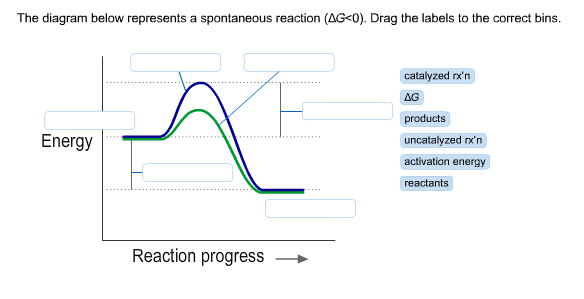
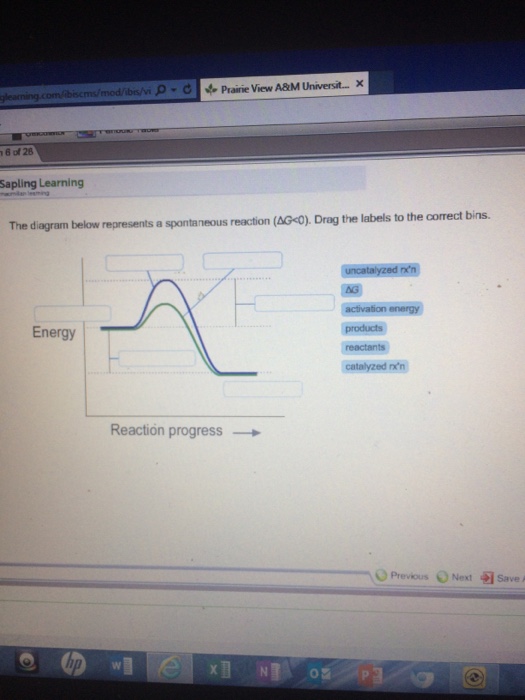
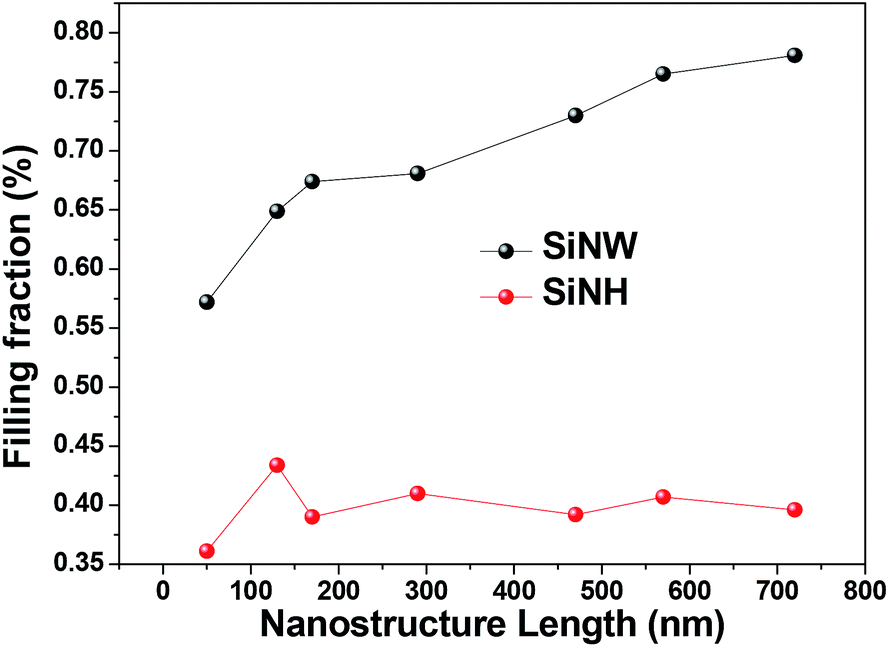
42 the diagram below represents a spontaneous reaction (δg
country songs about brown eyes? Determine the enthalpy for this reaction: mgcl2(s)+2naoh(aq)→mg(oh)2(s)+2nacl(aq) Determine the value of h such that the matrix is the augmented matrix of a consistent linear system; Write an expression whose value is the character at index 3 of the str associated with s. The diagram below represents a spontaneous reaction (δg° Cumalative Chem Final Flashcards | Quizlet In the diagram below, ... The equation below represents the equilibrium process of a saturated solution of Ca(OH)2 If the following changes are made to a saturated solution, what will be the effect on the ... The reaction is only spontaneous at low temperatures. b.
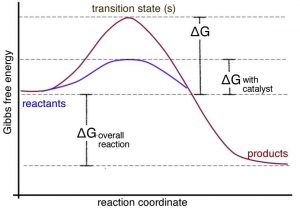
Gibbs free energy - chemguide ΔG = ΔH - TΔS. Remember that for a reaction to be feasible, ΔG has to be negative. ΔH could be negative (an exothermic reaction) or positive (an endothermic reaction). Similarly ΔS could be either positive or negative. There are four possible combinations of the signs of ΔH and ΔS. I want to look at those in turn.

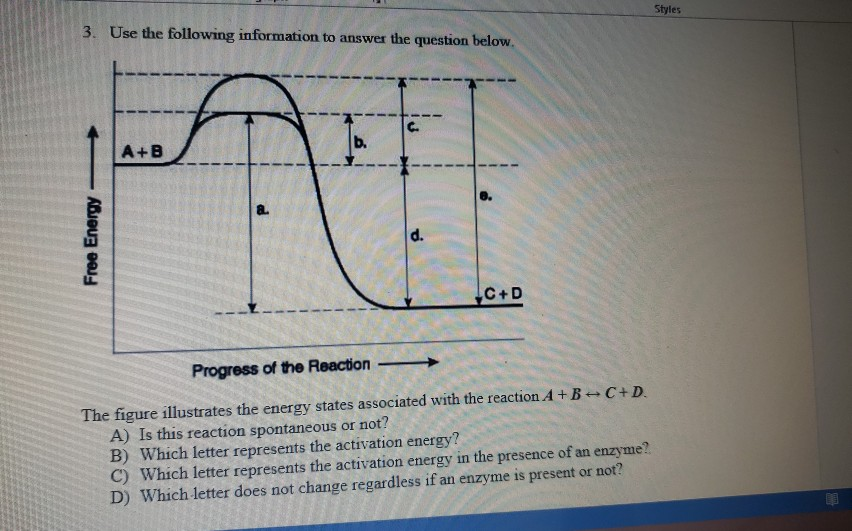
The diagram below represents a spontaneous reaction (δg
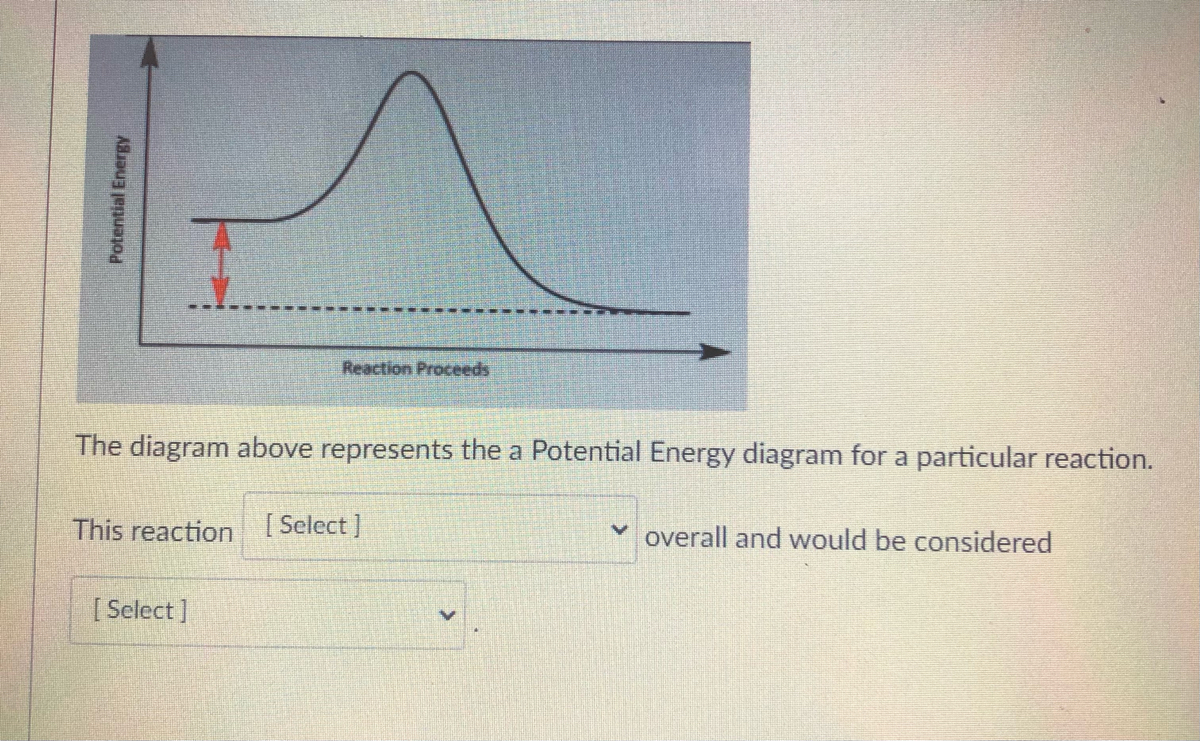
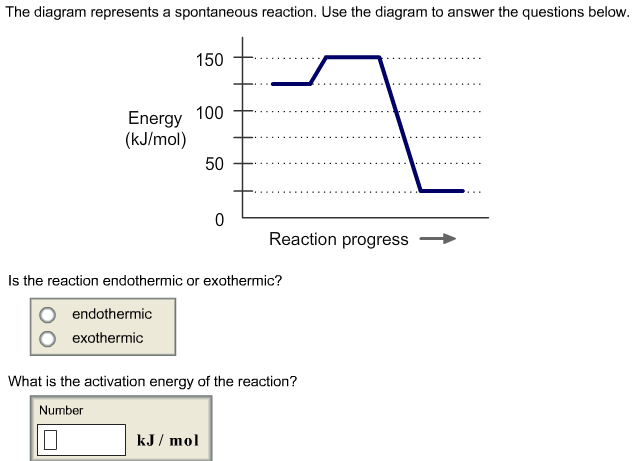
Bioenergetics 1 Flashcards - Quizlet If the ΔG'o of the reaction A → B is -40 kJ/mol, under standard conditions the reaction: will proceed spontaneously from left to right. The equation that best describes the relationship between Gibbs Free Energy (ΔG) and the equilibrium constant (Keq) of a reaction A--->B, under non-standard conditions (where standard conditions are: 25°C ... Solved The diagram represents a spontaneous reaction. Use ... The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? Question: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? Movies about ddlg? Secretary (2002) starring Maggie Gyllenhaal as Lee Holloway and James Spader as E. Edward Grey. Widget. 04:59. How To Solve Any Physics Problem. 09:10. 10 Rules of Learning Math Scientifically. 09:09. 8 Essential College Skills.
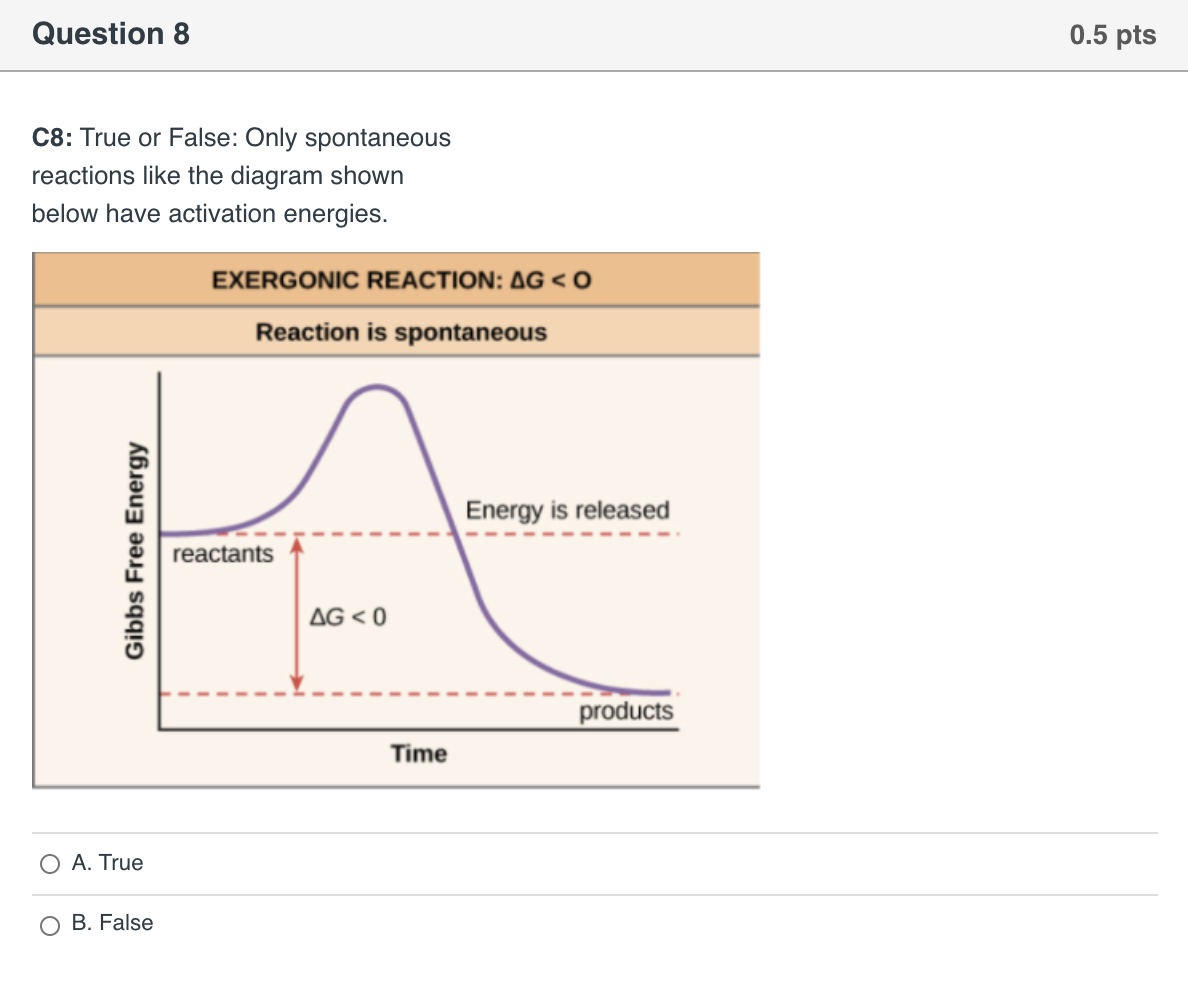
The diagram below represents a spontaneous reaction (δg. Solved The diagram represents a spontaneous reaction. Use ... The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? Expert Answer 100% (27 ratings) Solution: 1) It is an Exothermic reaction In exothermic reactions energy is released.In … View the full answer Previous question Next question Gibbs free energy - Wikipedia In thermodynamics, the Gibbs free energy (or Gibbs energy) is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure.The Gibbs free energy (=, measured in joules in SI) is the maximum amount of non-expansion work that can be extracted … PDF CHM 152 Exam 4 Review Ch. 18 19 KEY j. Based on your answers from c, g and h, determine if the following reaction is spontaneous. Explain. 2Ag(s) + Cu2+(aq) → Cu(s) + 2Ag+(aq) No, the reaction a written above is not spontaneous. Based on the spontaneous reaction written in part c, Cu(s) is more likely to give up electrons and Ag+(aq) is more likely to accept electrons. CHEM 152 UNIT III Flashcards | Quizlet The spontaneous redox reaction in a voltaic cell has _____ A) a negative value of Ecell and a negative value of ΔG. B) a positive value of Ecell and a positive value of ΔG. C) a negative value of Ecell and a positive value of ΔG. D) a positive value of Ecell and a negative value of ΔG. E) a positive value of Ecell and a value of zero for ΔG.
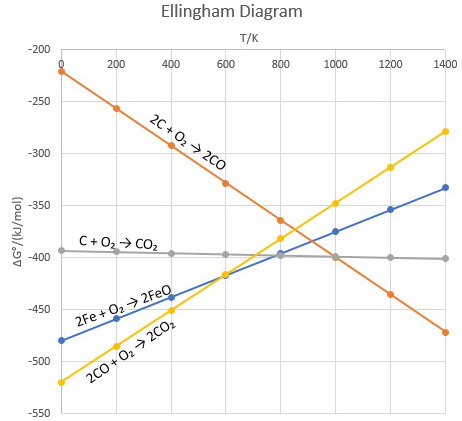
Solved The diagram below represents a spontaneous reaction ... 100% (22 ratings) Left side must include REACTANTS right side (on the bottom) must be products, since they must be accordingly to the r …. View the full answer. Transcribed image text: The diagram below represents a spontaneous reaction (deltaG < 0). Drag the labels to the correct bins. The reaction SO2(g) + 2H2S(g) ⇌ 3S(s) + 2H... | Clutch Prep The reaction SO 2 (g) + 2H 2 S(g) ⇌ 3S(s) + 2H 2 O(g) is the basis of a suggested method for removal of SO 2 from power-plant gases. The standard free energy of each substance are ΔG f °S(s) = 0 kJ/mol . ΔG f °H 2 O(g) = -228.57 kJ/mol . ΔG f °SO 2 (g) = -300.4 kJ/mol. ΔG f ° H 2 S (g) = -33.01 kJ/mol. What is the equilibrium constant for the reaction at 298K? In principle, is this ... What is δg for this reaction?, the sign of the standard ... 1) it is the amount of energy required to distort the nuclear configuration of the reactants into the nuclear configuration of the products without electron transfer occurring > 2zn + o2 2zno;δg^o = oscillations redox reactions limits and derivatives motion in a plane mechanical properties of fluids. class 12 atoms chemical kinetics moving … Thermodynamics Flashcards - Quizlet Classify each of the following processes as spontaneous or nonspontaneous. I. H2O(l) → H2O(g) T = 25°C, vessel open to atmosphere with 50% relative humidity II. H2O(s) → H2O(l) T = 25°C, P = 1 atm I and II are both spontaneous. The reaction A(g) → B(g) is spontaneous under standard conditions. Which of the following statements must be true? I.
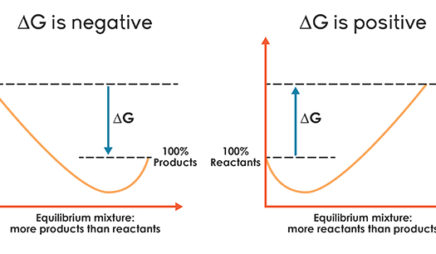
Gibbs Free Energy | Boundless Chemistry - Lumen Learning Recall the condition for spontaneous change: ΔG = ΔH - TΔS < 0. where ΔG = change in Gibbs free energy, ΔH = change in enthalpy, T = absolute temperature, and ΔS = change in entropy. It is apparent that the temperature dependence of ΔG depends almost entirely on the entropy change associated with the process. Gibbs Free Energy - Definition, Equations, 2nd Law of ... ΔG > 0; the reaction is non-spontaneous and endergonic. ΔG < 0; the reaction is spontaneous and exergonic. ΔG = 0; reaction is at equilibrium. Note: According to the second law of thermodynamics entropy of the universe always increases for a spontaneous process. ΔG determines the direction and extent of chemical change. CHEM 4311 HW4 *in progress collab Flashcards - Quizlet Activity is defined as a matter of convenience to make the equations with real gases and solutions easier to solve. To use molecular-level diagrams to predict the signs of thermodynamic properties. The equations ΔG∘=−RTlnKΔG∘=−RTlnK and ΔG=ΔH−TΔSΔG=ΔH−TΔS Chem 180 Exam 3 Flashcards - Quizlet The image represents a spontaneous, gaseous reaction at a constant temperature T K. Predict whether ΔH, ΔS, and ΔG for this reaction are positive, negative, or zero. Acetylene, C2H2, can be converted to ethane, C2H6, by a process known as hydrogenation.
Chapter 3 Study Questions Flashcards - Quizlet ΔG° indicates the change in the standard free energy as a reactant is converted to product. Given what you know about these values, which reaction below is the most favorable? a. ADP + Pi → ATP (ΔG° = +7.3 kcal/mole) b. glucose 1-phosphate → glucose 6-phosphate (ΔG° = −1.7 kcal/mole) c. glucose + fructose → sucrose (ΔG° = +5.5 kcal/mole)
Answered: Consider the weak acid H2A and its… | bartleby Which diagram below represents a buffer solution? Explain your answer. Transcribed Image Text: Consider the weak acid H,A and its conjugate base HA-. Which diagram below represents a buffer solution? Explain your answer. = H2A = HA- A В Expert Solution Want to see the full answer? Check out a sample Q&A here See Solution
Solved The diagram below represents a spontaneous reaction ... Question: The diagram below represents a spontaneous reaction (deltaG degree < 0). Drag the labels to the correct bins. uncatalyzed rx'n Delta G degree standard free E of activation products reactants catalyzed rx?n This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (61 ratings)
What does the x represent on a motion map Determine the enthalpy for this reaction: mgcl2(s)+2naoh(aq)→mg(oh)2(s)+2nacl(aq) Determine the value of h such that the matrix is the augmented matrix of a consistent linear system; Write an expression whose value is the character at index 3 of the str associated with s. The diagram below represents a spontaneous reaction (δg°
Movies about ddlg? Secretary (2002) starring Maggie Gyllenhaal as Lee Holloway and James Spader as E. Edward Grey. Widget. 04:59. How To Solve Any Physics Problem. 09:10. 10 Rules of Learning Math Scientifically. 09:09. 8 Essential College Skills.
Solved The diagram represents a spontaneous reaction. Use ... The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? Question: The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or exothermic?
Bioenergetics 1 Flashcards - Quizlet If the ΔG'o of the reaction A → B is -40 kJ/mol, under standard conditions the reaction: will proceed spontaneously from left to right. The equation that best describes the relationship between Gibbs Free Energy (ΔG) and the equilibrium constant (Keq) of a reaction A--->B, under non-standard conditions (where standard conditions are: 25°C ...



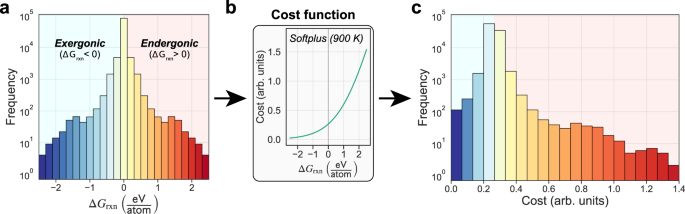

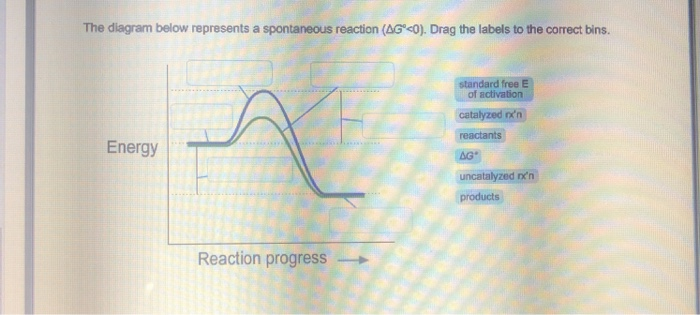
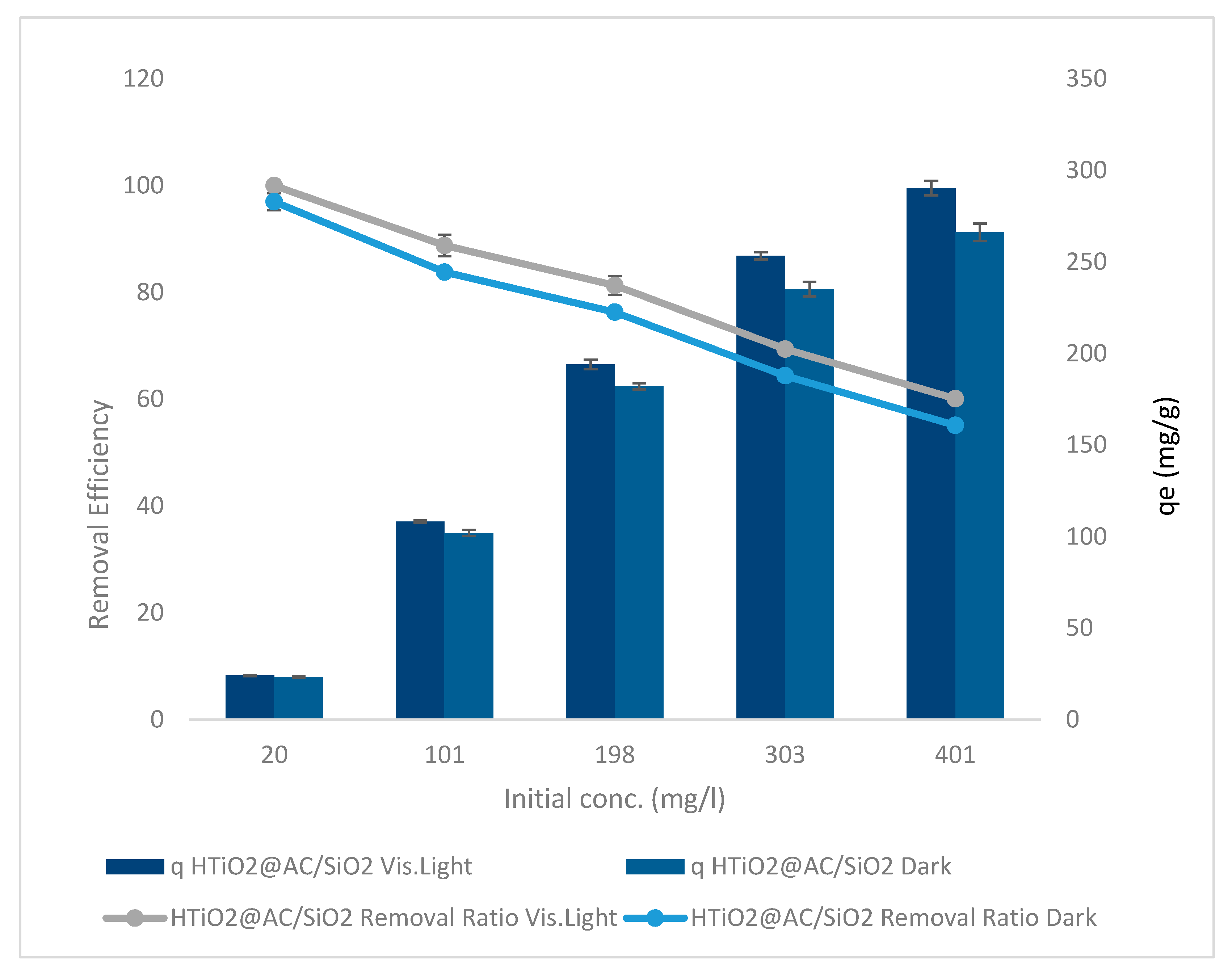







:max_bytes(150000):strip_icc()/endergonic-vs-exergonic-609258_final-2904b2c359574dfcb65a9fca2d54179a.png)

0 Response to "42 the diagram below represents a spontaneous reaction (δg"
Post a Comment