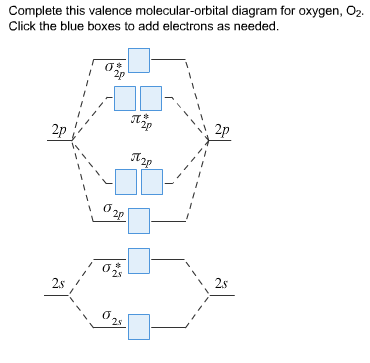
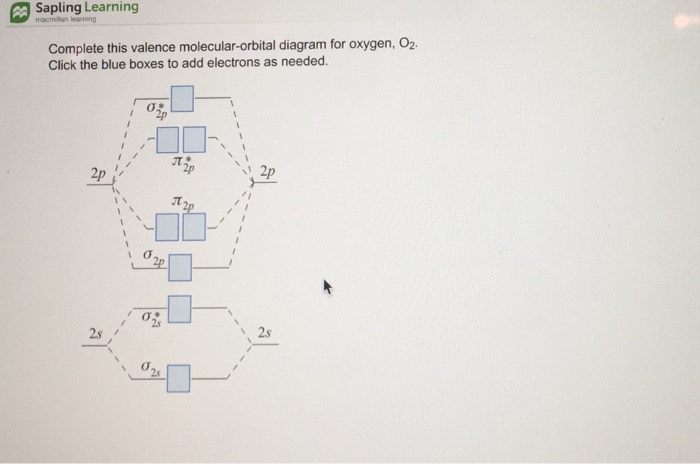
45 complete this valence molecular-orbital diagram for oxygen o2
Complete this valence molecular-orbital diagram for ... The total number of electrons present in O 2 {{\rm{O}}_2} O2 molecule are 16. Thus, the electronic configuration for combination of two atomic orbitals of ...1 answer · Top answer: Concepts and reason The concept used to solve this problem is based on molecular orbital diagram. A molecular orbital diagram is used to explain ... MO Orbital Lab - Part I: MO diagram of oxygen molecule, O2 ... Hydrogen only needs one sigma bond to complete their valence shell. In result, two sigma bonds connect to the oxygen molecule, making water. Since there are two sigma bonds, the maximum degeneracy for the water molecule is 1-fold. From the model, we don't get any of the same energy levels for any of the molecular orbitals.
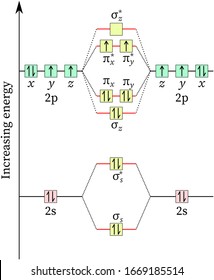
Special Case of Highly Electronegative Elements Atomic oxygen has 6 valence electrons and 4 valence orbitals (2s, 2p x, 2p y, and 2p z). We can draw a Lewis structure of molecular oxygen with a double bond between the oxygen atoms and 2 non-bonding pairs of electrons on each atom. However, experimentally we can determine that O 2 has 2 unpaired electrons. The Lewis structure seems to be ...

Complete this valence molecular-orbital diagram for oxygen o2
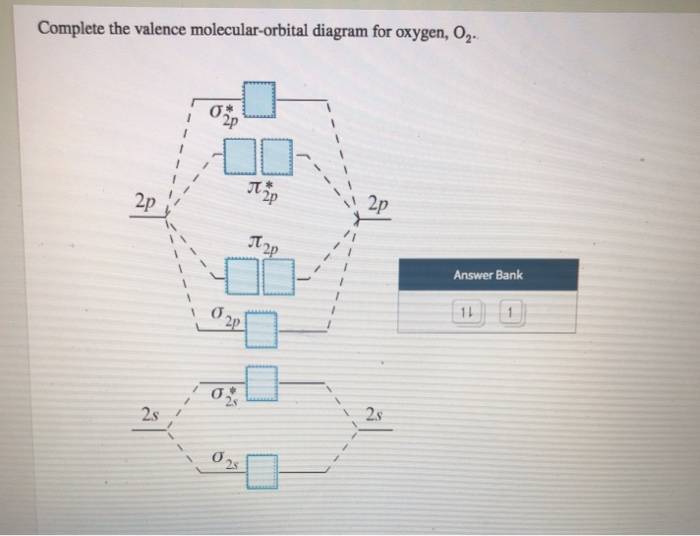
Explain the formation of O2 molecule using molecular ... The energy of σ 2 p z molecular orbital is greater than and molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behavior of the following species: N 2 , N 2 + , N 2 − , N 2 2 + Solved Complete the valence molecular-orbital diagram for ... Electronic configuration of Oxygen is So in O2 there is 12 valence e… View the full answer Transcribed image text : Complete the valence molecular-orbital diagram for oxygen, 0, 1,00 JT Žp 2p Answer Bank 02pm 21 Os Draw the valence shell molecular orbital diagram of oxygen ... Click here to get an answer to your question ✍️ Draw the valence shell molecular orbital diagram of oxygen molecule and predict its magnetic nature.Nov 19, 20191 answer · Top answer: The valence shell molecular orbital diagram of oxygen molecule is as shown. Oxygen molecule is paramagnetic due to presence of two unpaired electrons.
Complete this valence molecular-orbital diagram for oxygen o2. Solved complete this valence molecular orbital diagram for ... complete this valence molecular orbital diagram for oxygen O2 click the blue boxes to add electrons; Question: complete this valence molecular orbital diagram for oxygen O2 click the blue boxes to add electrons molecular orbital diagram for o2 - penguinskiclub.org Penguin Ski Club of New Hampshire. Located in Lincoln NH near Loon Mountain. Menu and widgets What is the orbital diagram of oxygen? Of oxygen is 8. Atoms can either donate or receive electrons only in valence shell. 8 electrons in outermost shell is considered the most stable state of an atom as the shell will be fully occupied. The valency of oxygen is 2 as its electronic configuration is 2,6 and it need 2 electron to complete their octet. Why is O2 paramagnetic class 11 chemistry CBSE We can use the molecular orbital diagram of oxygen molecules to explain the paramagnetic behaviour of oxygen atoms. We also have to know that for the paramagnetic nature of a molecule, it should contain a minimum of one unpaired electron. Complete answer: The valence bond theory could not explain the paramagnetic nature of oxygen molecules.
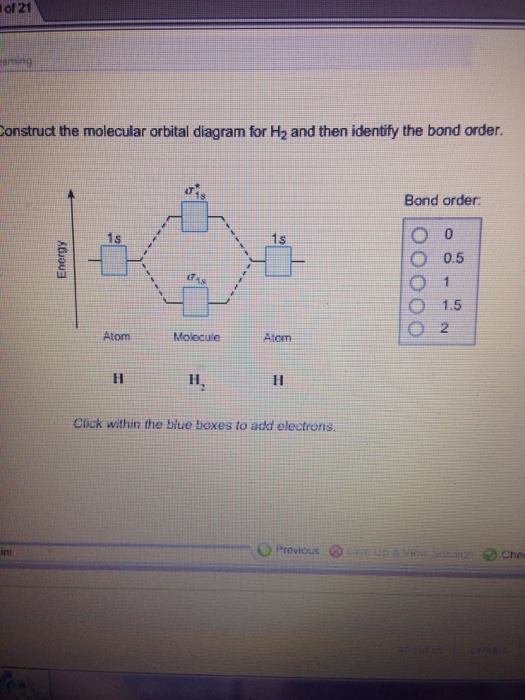
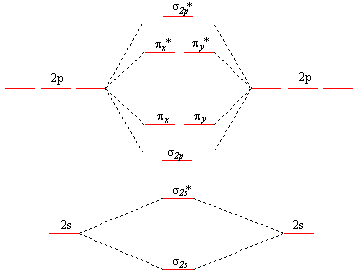
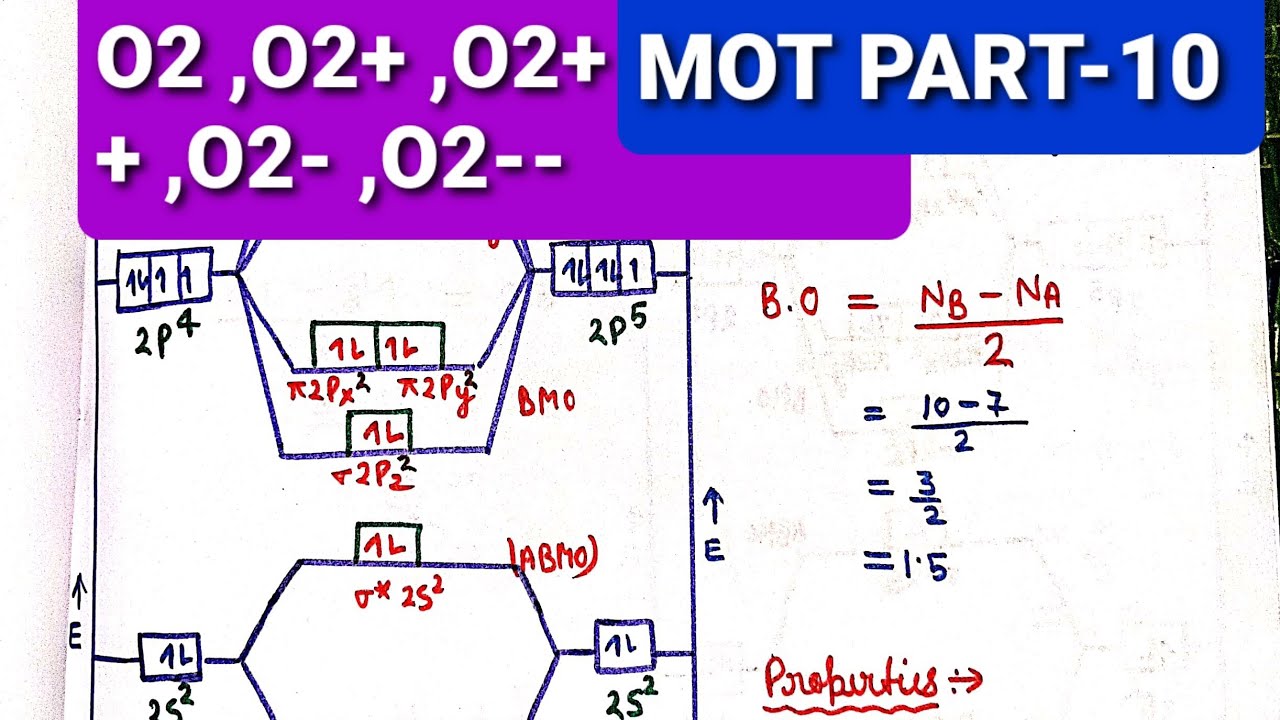
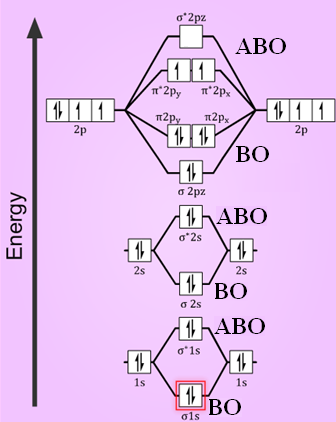
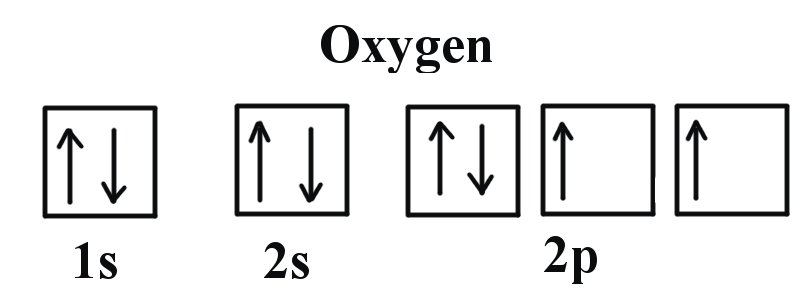
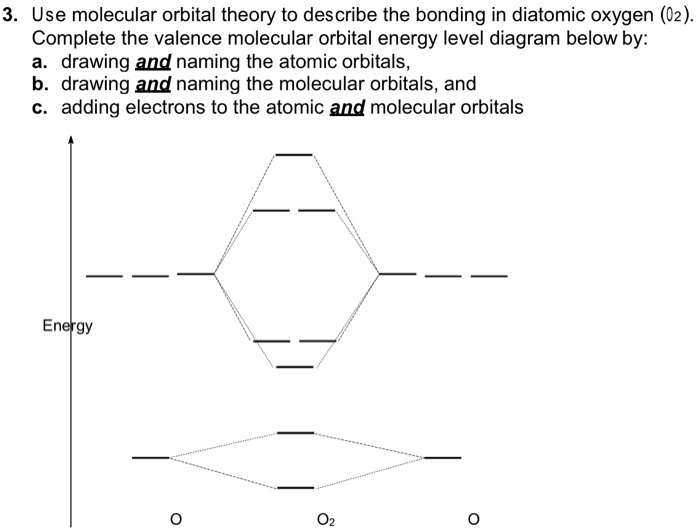
Molecular Orbital Theory - Purdue University molecular orbitals are those formed when valence-shell orbitals are combined. The molecular orbital diagram for an O2molecule would therefore ignore the 1selectrons on both oxygen atoms and concentrate on the interactions between the 2sand 2pvalence orbitals. Molecular Orbitals of the Second Energy Level Oxygen(O) electron configuration and orbital diagram Orbital Diagram for Oxygen (O) Oxide ion (O 2-) electron configuration Ground state electron configuration of oxygen is 1s 2 2s 2 2p x2 2p y1 2p z1. This electron configuration shows that the last shell of oxygen has six electrons. In this case, the valence electrons of oxygen are six. Complete this valence molecular-orbital diagram for oxygen ... Complete the valence molecular-orbital diagram for oxygen, O, Answer Bank. Complete the valence molecular-orbital diagram for oxygen, O, Answer Bank Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click... Construct the molecular orbital diagram for He2 and then identify the bond order. 39 molecular orbital diagram for o2 2- - Diagram For You - Quora Aug 25, 2017 — The Molecular orbital diagram for O2 is like this: As you can see the oxygen molecule has two unpaired electrons in the lower π* ant-bonding states. CHAPTER 5: MOLECULAR ORBITALS The Lewis structures have an unpaired electron and an average bond order of 1.5.
Molecular Orbital (MO) Diagram of O2 - YouTube Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*... O2 Lewis Structure, Molecular Geometry, and Hybridization The Lewis diagram of O2 shows two oxygen atoms having twelve dots, of valence electrons. Where six are arranged, around each oxygen atom in a way that one side has four valence electrons. These four valence electrons form two shared pairs of covalent bonds, providing a stable structure to the oxygen molecule. PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Solved Complete this valence molecular-orbital diagram for ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Complete this valence molecular-orbital diagram for oxygen, O2. Click the blue boxes to add electrons as needed.
Why is Valency of oxygen 2? Molecular oxygen (dioxygen) is a diradical containing two unpaired electrons, and superoxide results from the addition of an electron which fills one of the two degenerate molecular orbitals, leaving a charged ionic species with a single unpaired electron and a net negative charge of −1.
Draw the molecular orbital diagram of dioxygen and class ... In the molecular orbital hypothesis there is an intricate clarification of the paramagnetic character of oxygen. In the sub-atomic orbitals hypothesis, arrangement of the sub-atomic orbitals depends on the LCAO estimation technique, whereby nuclear orbitals comparing the valence shell of two, just participates in the development of sub-atomic ...
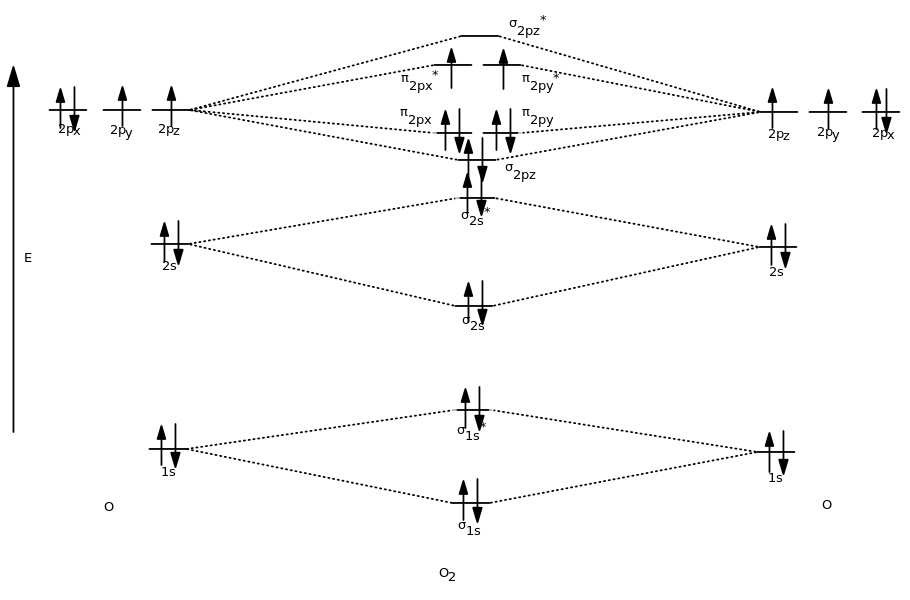
Explain the formation of O2 molecule using molecular class ... We know that Oxygen has atomic number = 8. Thus, the electronic configuration for an atom of oxygen in the ground state can be given as - $1{s^2}2{s^2}2{p^4}$ One atom of oxygen has 8 electrons. Thus, two atoms will possess 16 electrons i.e. Oxygen molecules will have 16 electrons. The molecular orbital diagram of an Oxygen molecule is as -
Molecular Orbital Theory - Chemistry Molecular orbital theory (MO theory) provides an explanation of chemical bonding that accounts for the paramagnetism of the oxygen molecule. It also explains the bonding in a number of other molecules, such as violations of the octet rule and more molecules with more complicated bonding (beyond the scope of this text) that are difficult to describe with Lewis structures.
Lecture18 (1) - Molecular Orbital Diagram for C2 Carbon ... Bonding in Heteronuclear Diatomics Molecular orbital diagrams for heteronuclear diatomics (AB) are constructed in a similar manner for homonuclear diatomics, but there is an important difference resulting from the fact that the energies of the atomic orbitals of A and B are different. Specifically, the MO diagram is skewed, with the atomic orbitals of the more electronegative element (A) being ...
What is the molecular orbital diagram of O2 and F2? - Quora Answer (1 of 6): Here is the solution, > * For O2 molecule, > * For F2 molecule, Thanks for reading.
Draw the valence shell molecular orbital diagram of oxygen ... Click here to get an answer to your question ✍️ Draw the valence shell molecular orbital diagram of oxygen molecule and predict its magnetic nature.Nov 19, 20191 answer · Top answer: The valence shell molecular orbital diagram of oxygen molecule is as shown. Oxygen molecule is paramagnetic due to presence of two unpaired electrons.
Solved Complete the valence molecular-orbital diagram for ... Electronic configuration of Oxygen is So in O2 there is 12 valence e… View the full answer Transcribed image text : Complete the valence molecular-orbital diagram for oxygen, 0, 1,00 JT Žp 2p Answer Bank 02pm 21 Os
Explain the formation of O2 molecule using molecular ... The energy of σ 2 p z molecular orbital is greater than and molecular orbitals in nitrogen molecule. Write the complete sequence of energy levels in the increasing order of energy in the molecule. Compare the relative stability and the magnetic behavior of the following species: N 2 , N 2 + , N 2 − , N 2 2 +
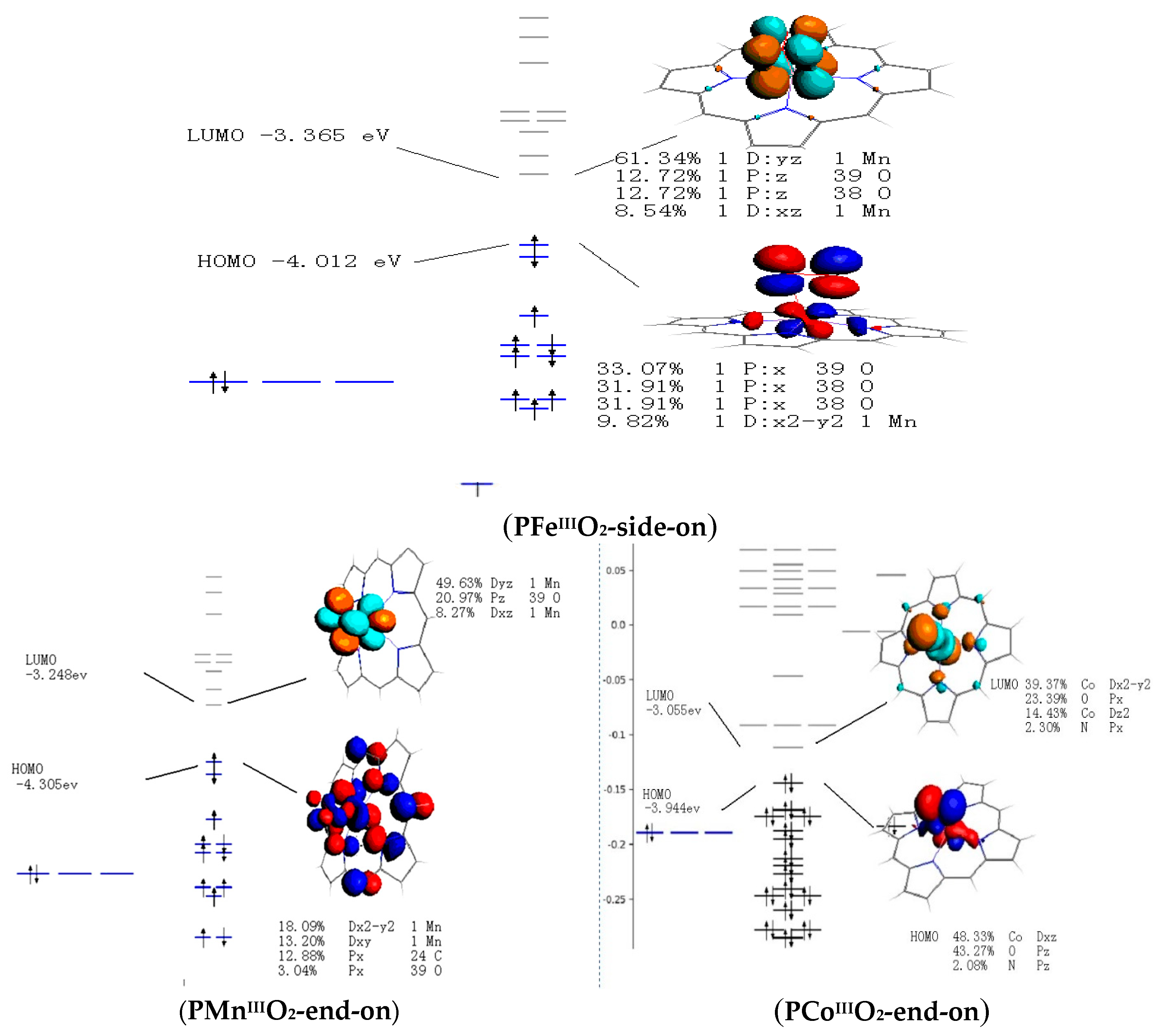




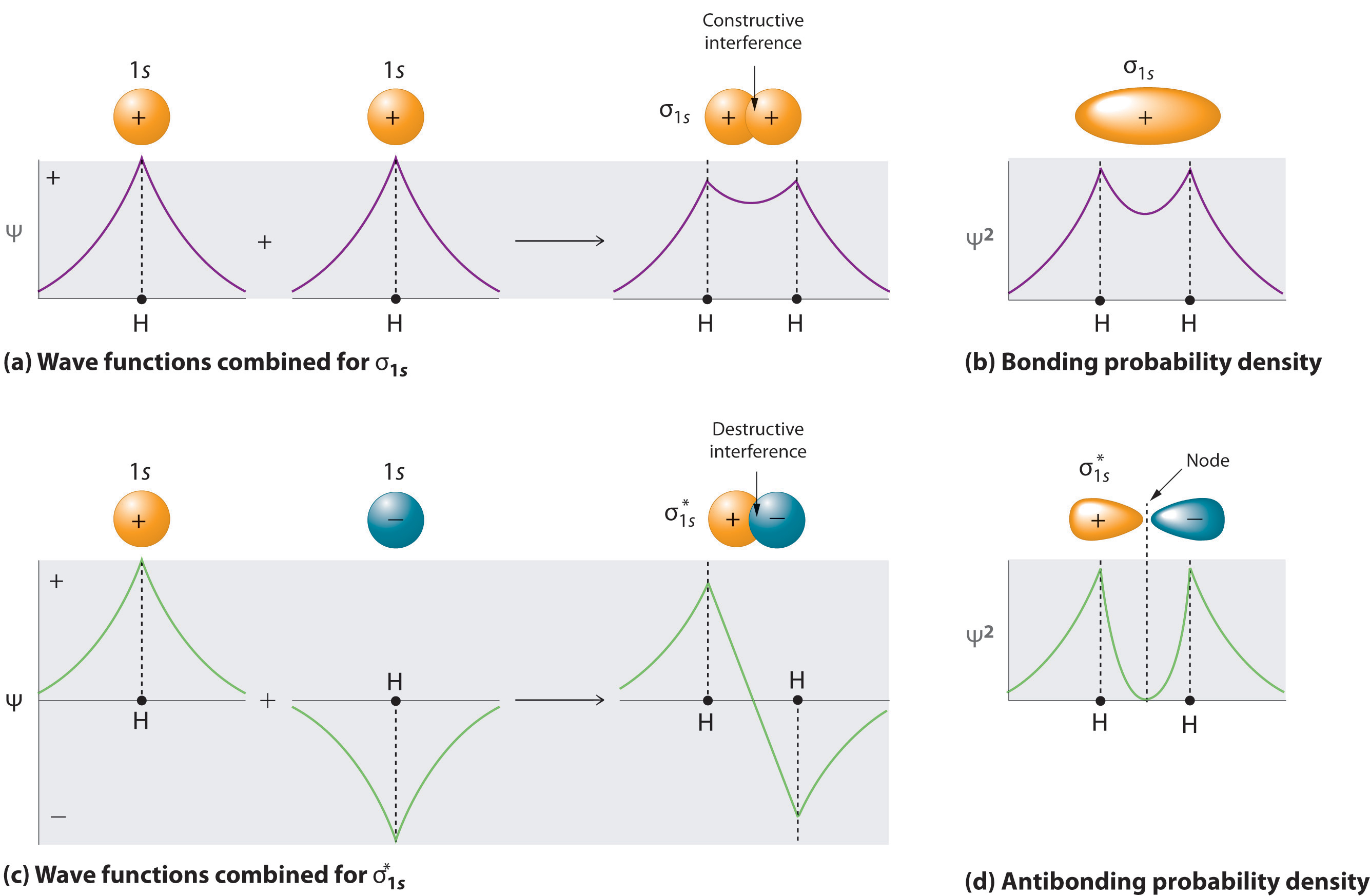

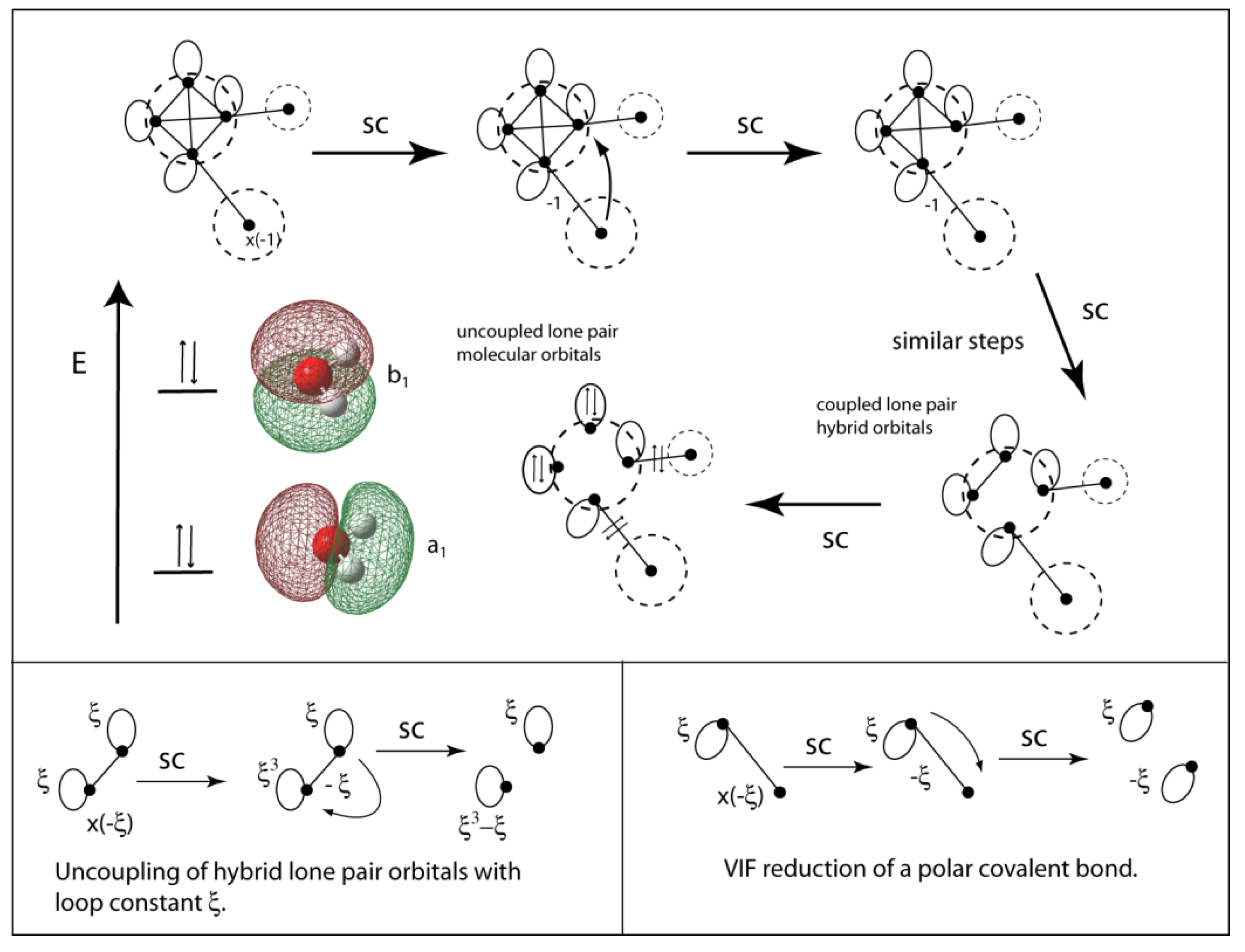


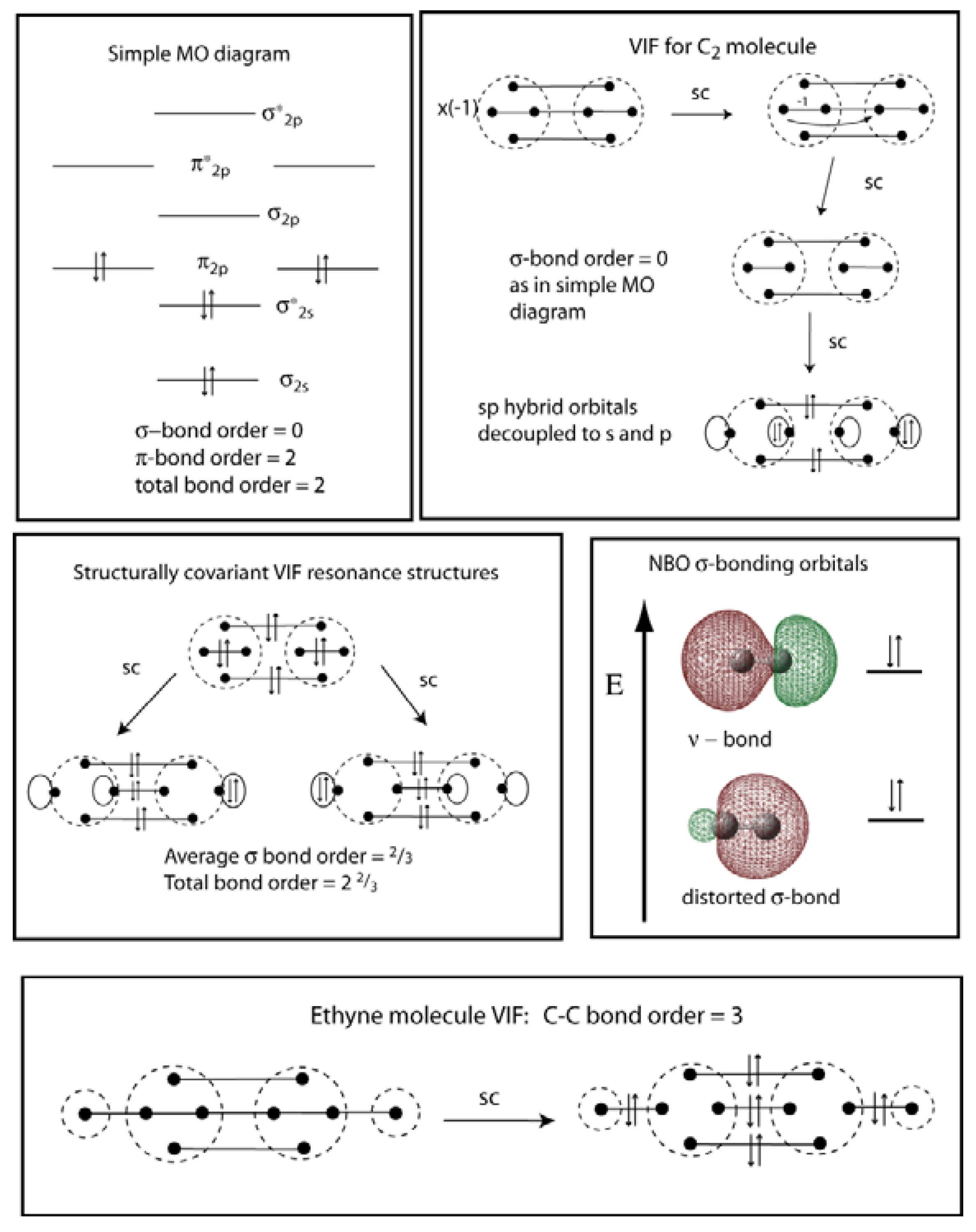














0 Response to "45 complete this valence molecular-orbital diagram for oxygen o2"
Post a Comment