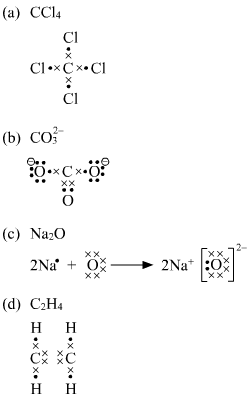
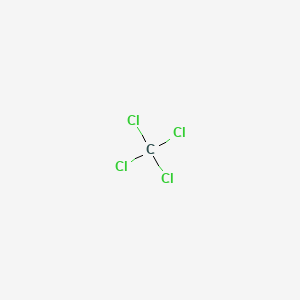
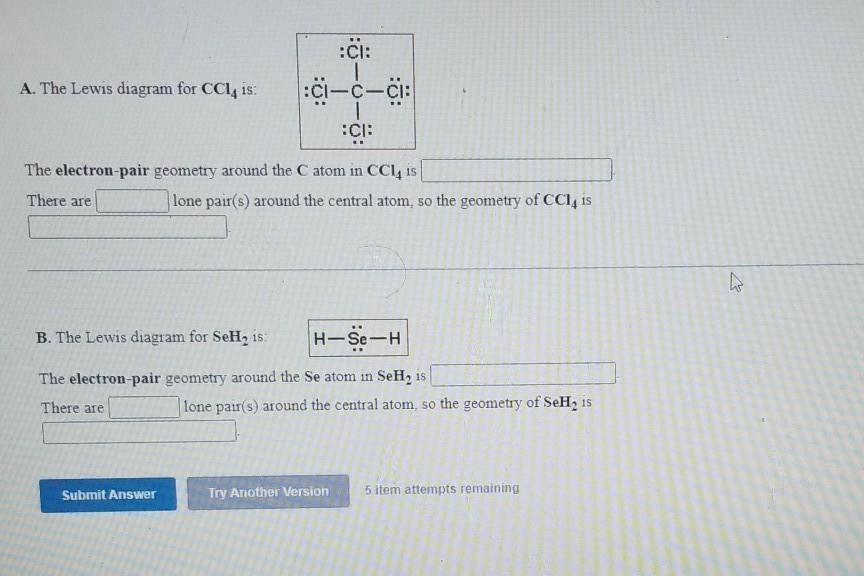
43 lewis diagram for ccl4
C2H4 Lewis Structure, Molecular Geometry, Hybridization ... C2H4 Lewis Structure The electron dot structure, widely known as Lewis Structure, is a skeletal diagrammatic representation of a molecule taking into account the constituent atoms and the valence shell electrons. What is the molecular geometry of CCl4? Draw its VSEPR and ... Explanation: Lewis Structure Here are the steps that I follow when drawing a Lewis structure. 1. Decide which atom is the central atom in the structure. That will be the least electronegative atom ( C ). 2. Draw a skeleton structure in which the other atoms are single-bonded to the central atom — a C atom with four Cl atoms attached to it. 3.
CCL4 Molecular Geometry, Lewis Structure, Hybridization ... For the Lewis structure of CCl4 first, let's calculate the total valence electrons. Carbon has four valence electrons and each Chlorine atom has seven valence electrons. As there are four molecules of Chlorine, we will calculate the number of valence electrons accordingly. = 4 + (4*7) = 4 + 28 = 32 valence electrons
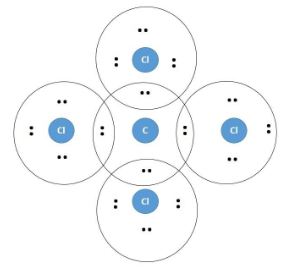
Lewis diagram for ccl4
CCl4 Lewis Structure - Science Trends So the total number of electrons in our diagram of CCl 4 should be: 1 (4)+4 (7) = 32 electrons. Step 2. Sketch out a skeleton of the compound's atomic structure. Next up is to figure out the atomic organization of the compound. quizlet.com › 563681428 › chemistry-unit-6-flash-cardsChemistry, Unit 6 Flashcards - Quizlet 16) A solution containing 94.4 mL of carbon tetrachloride, CCl4, dissolved in 250.0 mL of nitrobenzene, C6H5NO2, was placed in the freezer, which dropped the temperature to 0°C. Did the solution freeze at that temperature? If so, at what temperature? The density of CCl4 is 1.59g/cm3. The density of C6H5NO2 is 1.2 g/cm3. CCl4 Lewis Structure, Molecular Geometry, Hybridization ... MO Diagram of CCl4 A MO diagram is nothing but a representation of bonds that are formed within the atoms to form a compound. This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4's and CH4 MO diagram looks like.
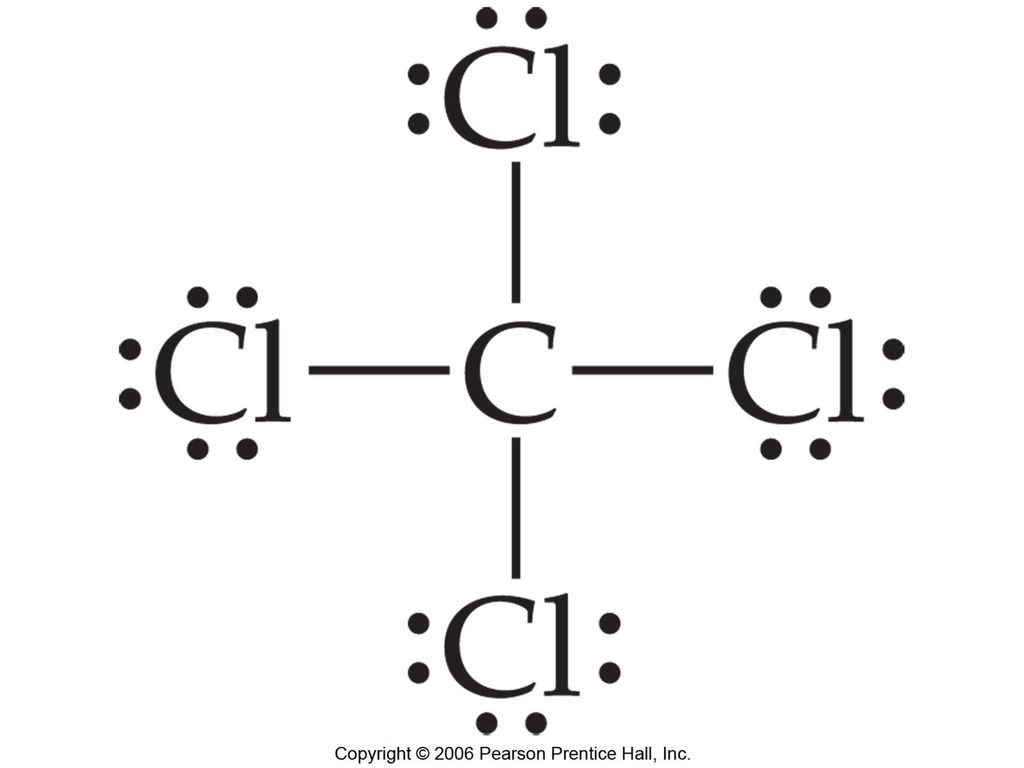
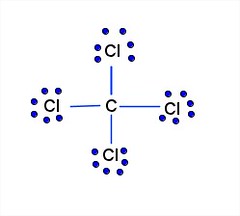
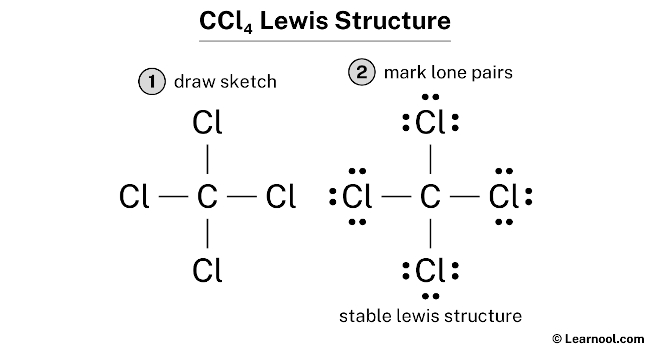
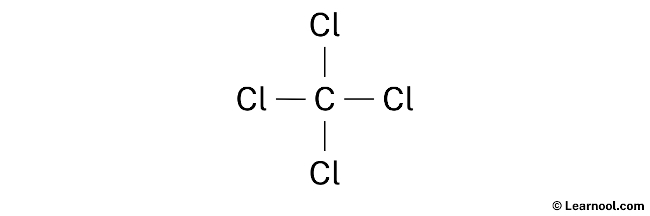
Lewis diagram for ccl4. CCl4 Lewis Structure - Learnool CCl 4 (carbon tetrachloride) has one carbon atom and four chlorine atoms. In the lewis structure of CCl 4, there are four single bonds around the carbon atom, with four chlorine atoms attached to it, and on each chlorine atom, there are three lone pairs. Steps Here's how you can draw the CCl 4 lewis structure step by step. Step #1: draw sketch CCl4 Molecular Geometry - Science Education and Tutorials Use the formula below to find the lone pair on the CCl4 molecule's central carbon atom. L.P (C) = V.E (C) - N.A (C-Cl)/2 Lone pair on the central carbon atom = L.P (C) The core central carbon atom's valence electron = V.E (C) Number of C-Cl bonds = N.A (C-Cl) calculation for carbon atom lone pair in CCl4 molecule Lewis Structure Xecl4 The XeCl4 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the XeCl4 molecule. What are the approximate bond angles in XeCl_4. XeCl4 4 molecule is. The molecular shape is j square planar. An SF 2 molecule is polar or nonpolar. However the central xenon atom does not. topblogtenz.com › ethene-c2h4-lewis-dot-structureC2H4 lewis structure, molecular geometry, bond angle ... C2H4 Lewis structure contains four C-H bonds and one double bond in between two carbon atoms. No lone pair is present on the central or outer atom in the lewis structure of C2H4. The lewis dot structure of C2H4 is very easy to draw-Some steps need to follow for drawing the C2H4 Lewis dot structure. 1. Count total valence electron in C2H4
Ccl4 Lewis - chemteam writing lewis structures, solved ... Ccl4 Lewis - 13 images - lewis dot structure of ccl4 carbon tetrachloride youtube, 34 lewis dot diagram for ccl4 wiring diagram database, lewis structures ccl4 youtube, valence shell electron pair repulsion presentation chemistry, Solved Draw the Lewis structure of CCl4. Include all ... This problem has been solved! Draw the Lewis structure of CCl4. Include all the lone pairs. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Draw the Lewis structure of CC14. CF4 lewis structure, Molecular geometry, Polar or nonpolar ... The lewis diagram of CF4 is very similar to CCl4. Let's see step by step how to draw this in a very simple way with all explanations. Follow some steps for drawing the lewis dot structure of CF4 1. Count total valence electron in CF4 The electrons found in the outermost shell of an atom are called valence electrons. Cl4 Lewis Structure - lewis dot diagram for ccl4 free ... Cl4 Lewis Structure - 15 images - chemistry partner molecular geometry compounds with, ccl4 lewis structure how to draw the dot structure for, how to draw the lewis structure for pcl4 youtube, lewis structure of clo2 chemistry stack exchange,
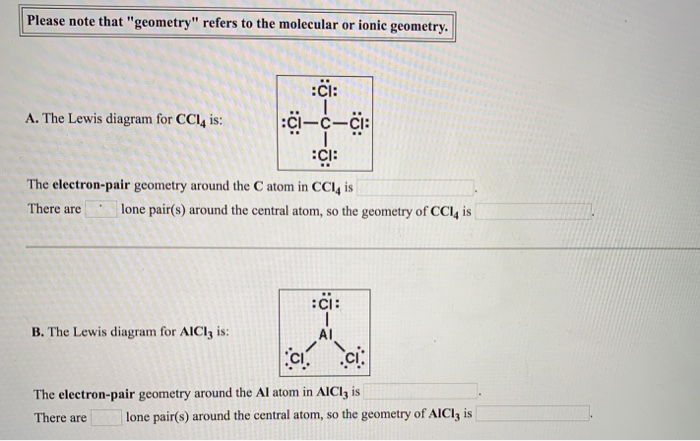
quizlet.com › 605114887 › chem-66-76-flash-cardsChem 6.6-7.6 Flashcards | Quizlet Consider the molecule CCl4. Each C-Cl bond in this molecule is _____ because the electronegativity difference between C and Cl is equal to 0.5. Since CCl4 is tetrahedral in shape and symmetrical, the individual bond dipoles _____ and the molecule is _____ overall. ClO4- Lewis Structure (Perchlorate ion) Lewis structure of ClO4- ion is drawn step by step in this tutorial. Total valence electrons of given by four oxygen atoms and,chlorine atom and negative charge are considered to draw the ClO4- lewis structure. Chlorine gives seven electrons to valence electrons. Lewis structure for CCl4? - Answers The Lewis dot structure for CCl4 starts with a C in the middle. Four dashes are drawn, one on each side, each connecting to a Cl atom. On the unconnected sides of the Cl atoms, there are two dots ... CCl4 Lewis Structure - How to Draw the Dot Structure for ... A step-by-step explanation of how to draw the CCl4 Lewis Dot Structure (Carbon tetrachloride). The Lewis structure for CCl4 is a commonly tested Lewis struc...
Ccl4 Lewis Structure - ccl4 lewis structure science trends ... Ccl4 Lewis Structure - 17 images - ccl4 lewis structure science trends, c2h2cl2 lewis structures, preparation of oxazolines containing an additional soft, carbon tetrachloride ccl4 lewis structure slidesharetrick,
techiescientist.com › sio2-lewis-structureSiO2 Lewis Structure, Molecular Geometry, Hybridization, and ... 1 day ago · SiO2 has a net dipole moment of zero. It has a linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp. The Silicon dioxide Lewis structure has a total of 16 valence electrons. In the Lewis dot structure of SiO2, the formal charge is zero. If you have any doubts, please feel free to ask in the comments ...
› questions-and-answers › identifyAnswered: Identify the excess reactant and how… | bartleby Apr 22, 2022 · A: Here vapour pressure of liquid CCl4 is 100 mm Hg at 296K .Here 0.261 g of CCl4 was placed in 400 ml… question_answer Q: Balance the following redox half-reactions
What is the Lewis dot structure for CCl4? - Answers The Lewis dot structure for CCl4 starts with a C in the middle. Four dashes are drawn, one on each side, each connecting to a Cl atom. On the unconnected sides of the Cl atoms, there are two dots ...
CCl4 Lewis Structure - YouTube Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry of carbon tetrachloride (CCl4)
Lewis structure calculator | Lewis structure generator The Lewis structure, proposed by Gilbert Newton Lewis, who introduced it for the first time in 1916, is a graphic representation of the sharing of electrons that occurs in chemical bonds between atoms of the same or different species. These bonds can be single, double, or triple.
Lewis Dot Structure and Polarity of CCl4 (Carbon ... Lewis Dot Structure and Polarity of CCl4 (Carbon Tetrachloride) Carbon tetrachloride, also known as tetrachloromethane, is a compound containing carbon and chlorine. It is an inorganic compound that is non-flammable. This ScienceStruck post provides you with the Lewis dot structure diagram and the polarity of carbon tetrachloride.
› 9569261 › Study_Guide_and(PDF) Study Guide and Solutions Manual to ... - Academia.edu Study Guide and Solutions Manual to Accompany T.W. Graham Solomons / Craig B. Fryhle / Scott A. Snyder / Jon Antilla
How to draw a CCl4 Lewis Structure? - Science Education ... In a CCl4 Lewis Structure diagram, the carbon atom can be the centre atom. As a result, central carbon in the CCl4 Lewis Structure, with all four Chlorines arranged around the tetrahedral geometry. Step-3: Combining step1 and step2 to get step3 for CCl4 dot structure
Lewis Structure Of Xecl4 - aunitedkingdomfilm.com The XeCl4 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the XeCl4 molecule. Xenon Tetrachloride XeCl4 Lewis Dot Structure. What are the approximate bond angles in XeCl_4. The shape of the hybridization is octahedral. The molecular shape is j square planar. The Lewis structure of CCl4 is.
CCl4 Lewis Structure, Molecular Geometry, Hybridization ... MO Diagram of CCl4 A MO diagram is nothing but a representation of bonds that are formed within the atoms to form a compound. This diagram is based on Molecular orbital theory. With the help of a MO diagram, the existence of certain compounds can be explained. Here is the pictorial representation of how CCl4's and CH4 MO diagram looks like.
quizlet.com › 563681428 › chemistry-unit-6-flash-cardsChemistry, Unit 6 Flashcards - Quizlet 16) A solution containing 94.4 mL of carbon tetrachloride, CCl4, dissolved in 250.0 mL of nitrobenzene, C6H5NO2, was placed in the freezer, which dropped the temperature to 0°C. Did the solution freeze at that temperature? If so, at what temperature? The density of CCl4 is 1.59g/cm3. The density of C6H5NO2 is 1.2 g/cm3.
CCl4 Lewis Structure - Science Trends So the total number of electrons in our diagram of CCl 4 should be: 1 (4)+4 (7) = 32 electrons. Step 2. Sketch out a skeleton of the compound's atomic structure. Next up is to figure out the atomic organization of the compound.





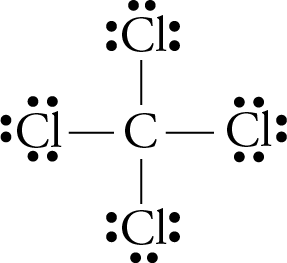
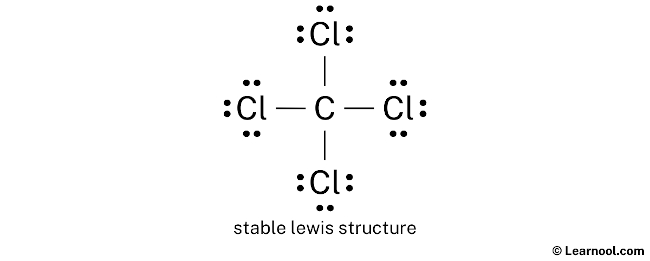
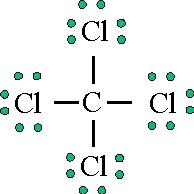


















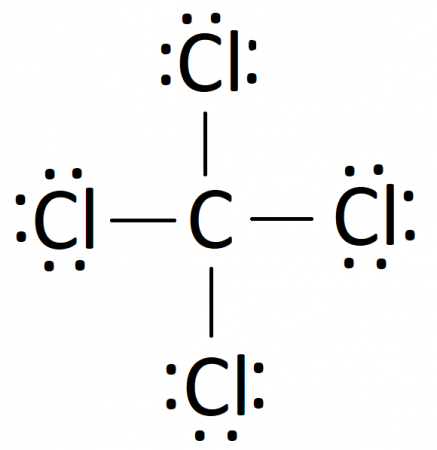

0 Response to "43 lewis diagram for ccl4"
Post a Comment