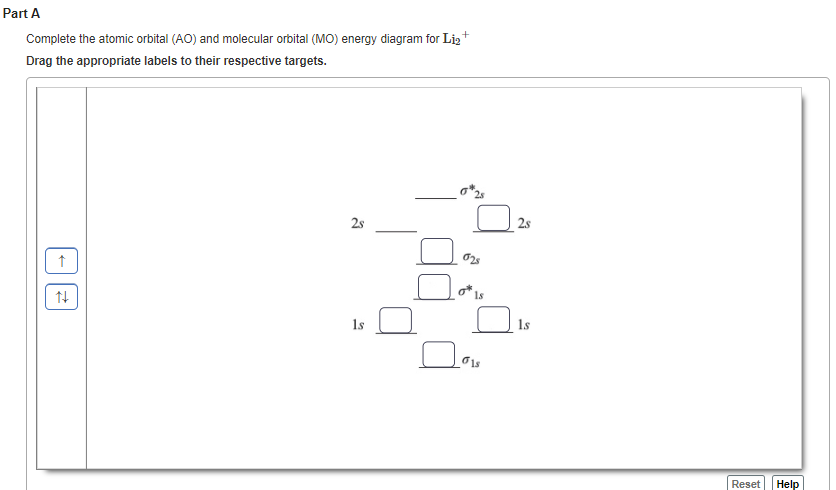
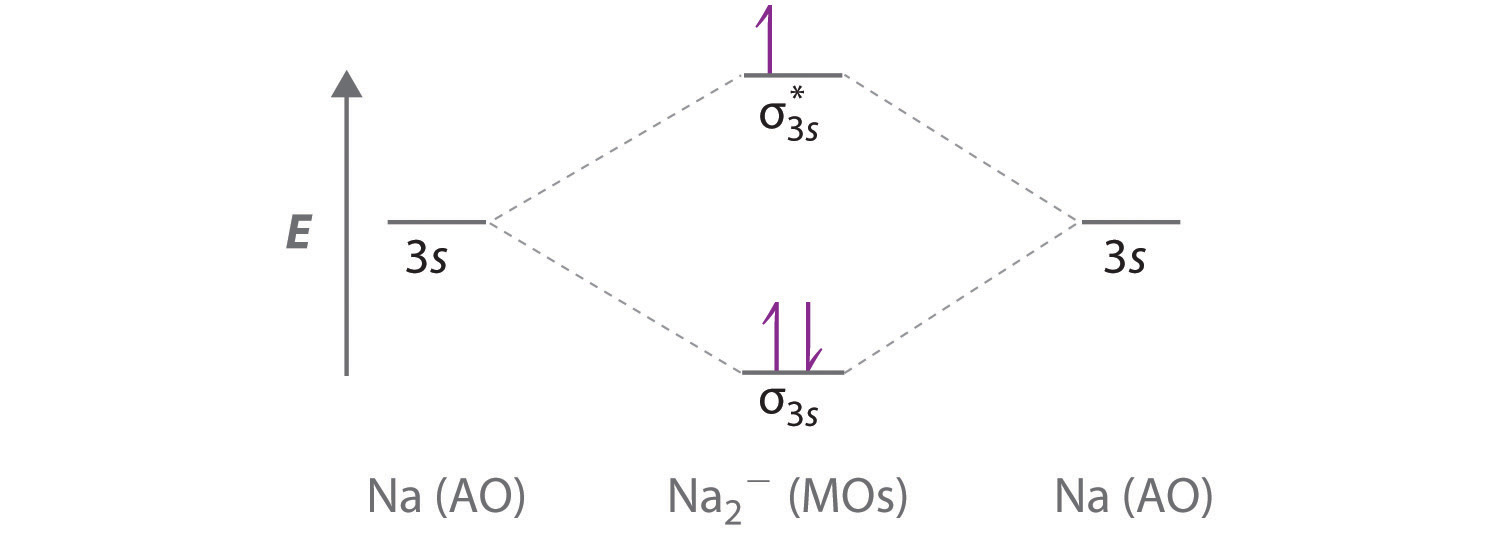
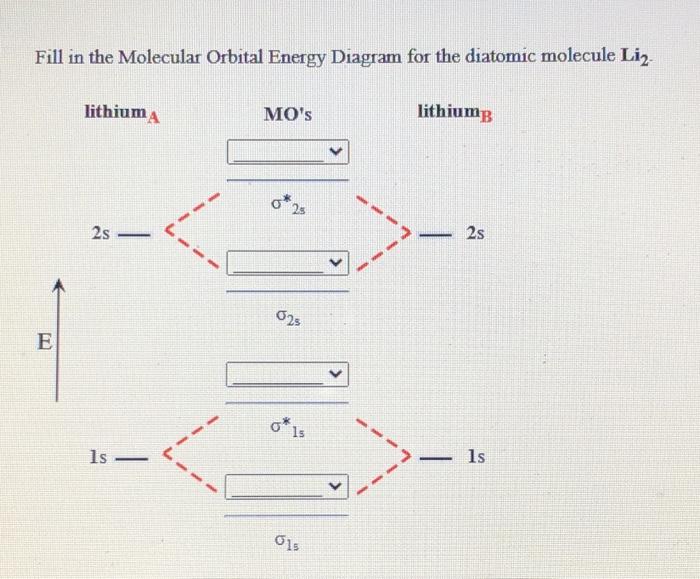
42 complete the ao and mo energy diagram for li2−
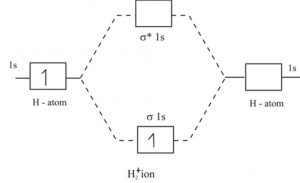
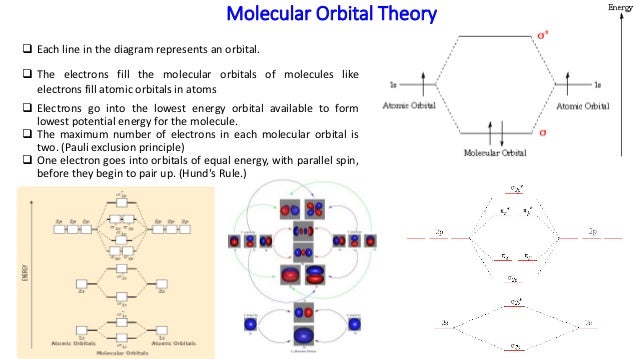
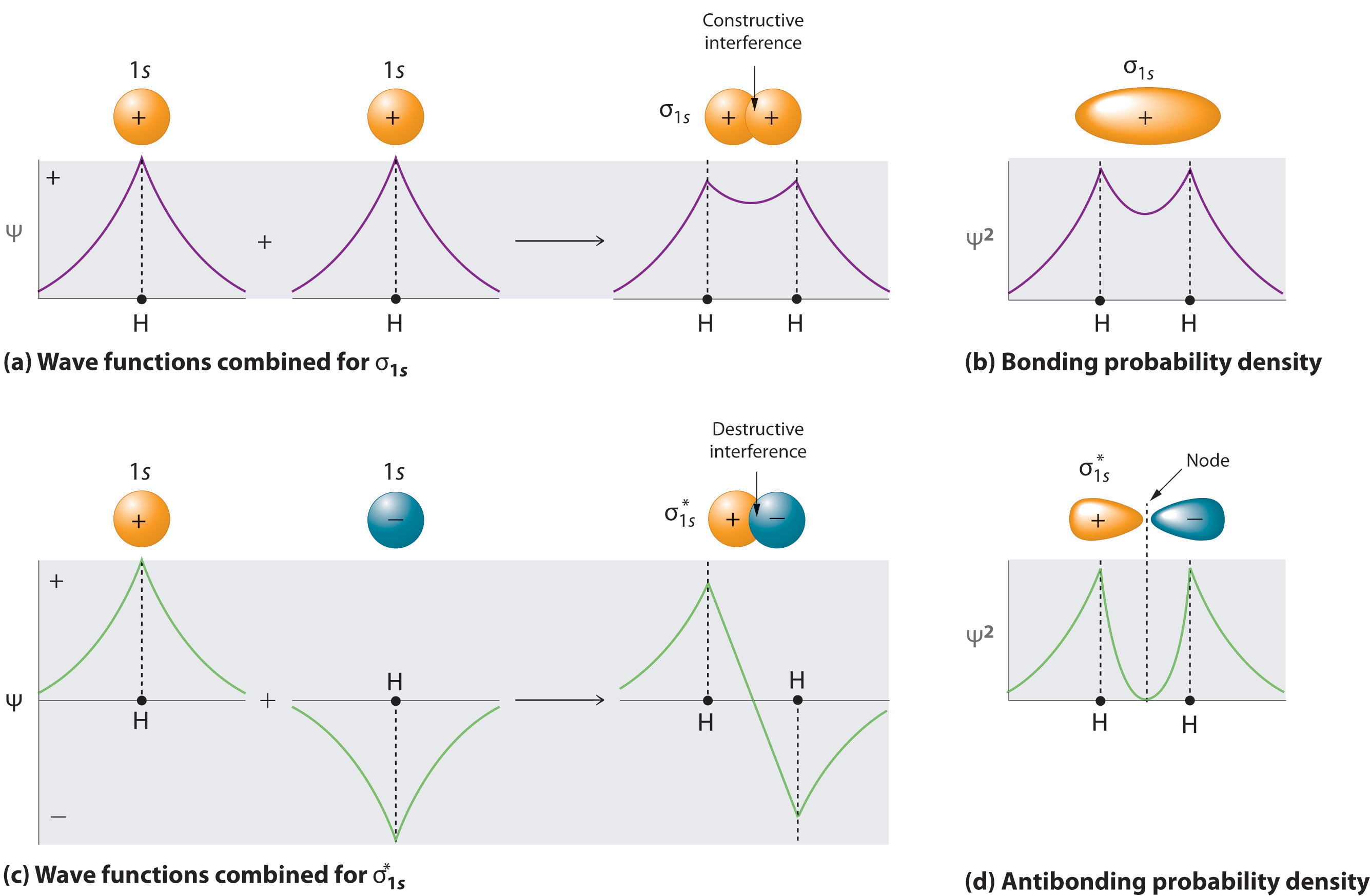
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ... Solid State ChemiStry and itS appliCation 2014 Anthony R. West
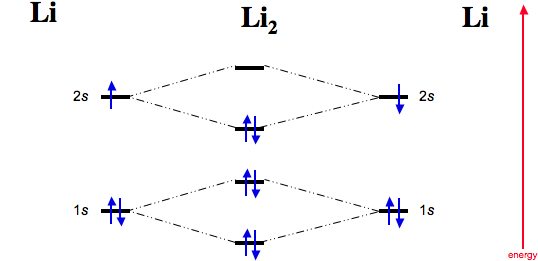
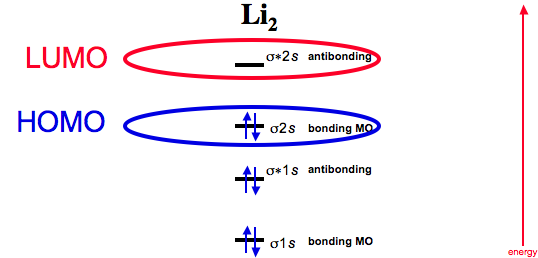
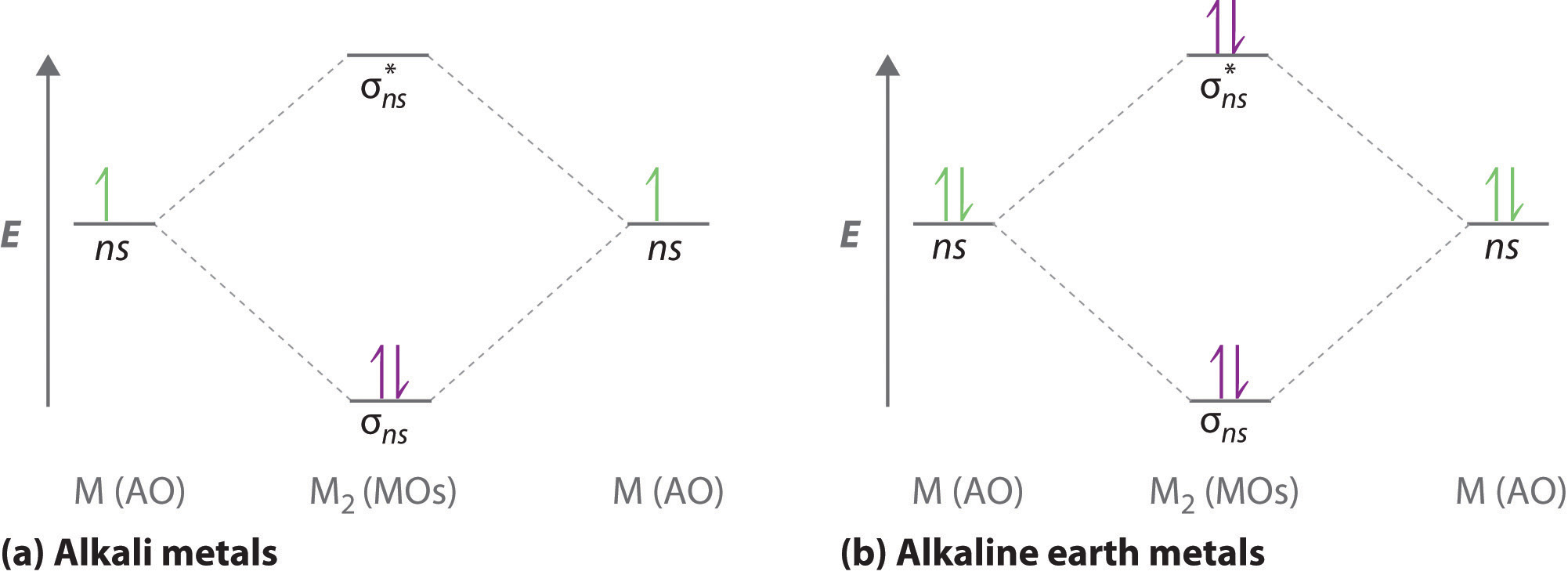
Practice energy diagrams for molecular orbital theory. Problem complete the atomic orbital ao and molecular orbital mo energy diagram for li 2. Solved part b complete the ao and mo energy diagram for l. σ bonding mo that is lower in energy than the constituent 2s aos and an antibonding σ mo that is at a higher energy than the 2s aos.

Complete the ao and mo energy diagram for li2−
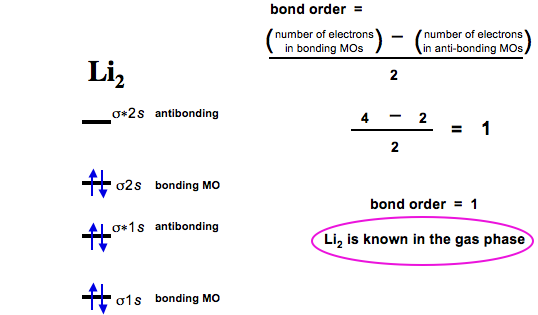
N b = 2 , Na =0. Bond order = 1. Positive value of bond order indicates that H 2 molecule is stable.. Bond order value of 1 means that two hydrogen atoms are connected by a single bond.. Greater value of bond order for H 2 molecule than H 2 + ion shows that two H 2 molecule is more stable than H 2 +.. Bond length of H 2 is smaller than that of H 2 + ion.. As no unpaired electron is present ... Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply 26 Complete The Ao And Mo Energy Diagram For Li2− Wiring. Click Images to Large View 26 Complete The Ao And Mo Energy Diagram For Li2− Wiring. Molecular Orbital Wikipedia. Click Images to Large View Molecular Orbital Wikipedia. Fileelectron Orbitalssvg Wikimedia Commons.
Complete the ao and mo energy diagram for li2−. Complete the ao and mo energy diagram for li2. Solved part b complete the ao and mo energy diagram for l. Problem complete the atomic orbital ao and molecular orbital mo energy diagram for li 2. Each mo can hold two es and hence for li2 the mo scheme is σ σ0 li2 is present the extent of 1 in lig. Use the drawing of the mo energy diagram to ... Explain. What is the bond order? Excuse for that I interfere … At me a similar situation. Get answers by asking now. The traditional chemical approaches, Lewis electron dot stru Complete the AO and MO energy diagram for Li2−. Assume the left AO comes from Li− and the right AO comes from Li. Question: Complete the AO and MO energy diagram for Li2−. Assume the left AO comes from Li− and the right AO comes from Li. Use the drawing of the mo energy diagram to predict the bond order of li2. Complete the ao and mo energy diagram for li2. Answer to 1complete the atomic orbital ao and molecular orbital mo energy diagram for li2 and li2. Each mo can hold two es and hence for li2 the mo scheme is σ σ0 li2 is present the extent of 1 in lig.
Molecular orbital diagram for ne2 2. 2s. I know ill need a 2p orbital but theres orbital mixing going on so i have 2 choices. Wiki User Answered 2012-03-05 06:41:57. Because According to molecular orbital theory O 2 + has 15 electrons &it has one electron in antibonding orbital. Side by Side Comparison – Homo vs Lumo in Tabular Form 5. C 2 c ... Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and. for each electron in a bonding MO, it adds 0.5 to the bond order, because more bonding ... 26 Complete The Ao And Mo Energy Diagram For Li2− Wiring. Click Images to Large View 26 Complete The Ao And Mo Energy Diagram For Li2− Wiring. Molecular Orbital Wikipedia. Click Images to Large View Molecular Orbital Wikipedia. Fileelectron Orbitalssvg Wikimedia Commons. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply
N b = 2 , Na =0. Bond order = 1. Positive value of bond order indicates that H 2 molecule is stable.. Bond order value of 1 means that two hydrogen atoms are connected by a single bond.. Greater value of bond order for H 2 molecule than H 2 + ion shows that two H 2 molecule is more stable than H 2 +.. Bond length of H 2 is smaller than that of H 2 + ion.. As no unpaired electron is present ...

1 Complete The Atomic Orbital Ao And Molecular Orbital Mo Energy Diagram For Li2 And Li2 Homeworklib
Solved Draw An Mo Energy Diagram And Predict The Bond Order Of Li2 And Li2 Do You Expect These Molecules To Exist In The Gas Phase
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11

Solved Chapter 10 Problem 42e Solution Masteringchemistry Standalone Access Card For Principles Of Chemistry 2nd Edition Chegg Com

How To Draw Molecular Orbital Diagram Of Li2 Li 2 Li2 Simplest Trick Chemistry Best Online Free Chemistry Class 9 12



















0 Response to "42 complete the ao and mo energy diagram for li2−"
Post a Comment