42 nitrogen atomic orbital diagram
Molecular orbital diagram for hydrogen: For a diatomic molecule, an MO diagram effectively shows the energetics of the bond between the two atoms, whose AO unbonded energies are shown on the sides. The unbonded energy levels are higher than those of the bound molecule, which is the energetically-favored configuration. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. diagram for CO2 in Figure can be used as a guide, with the orbitals of Be higher ...
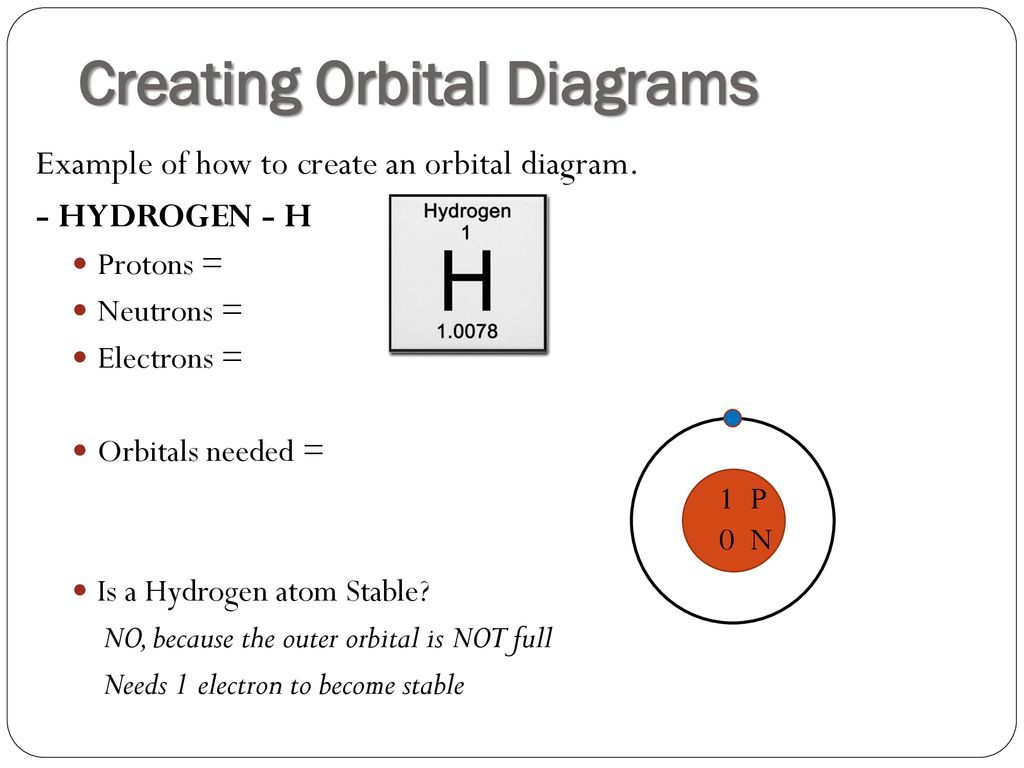
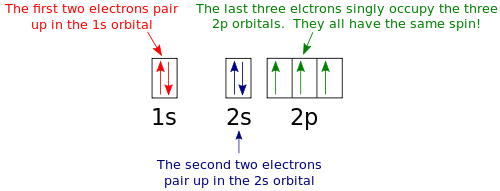
Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s.

Nitrogen atomic orbital diagram
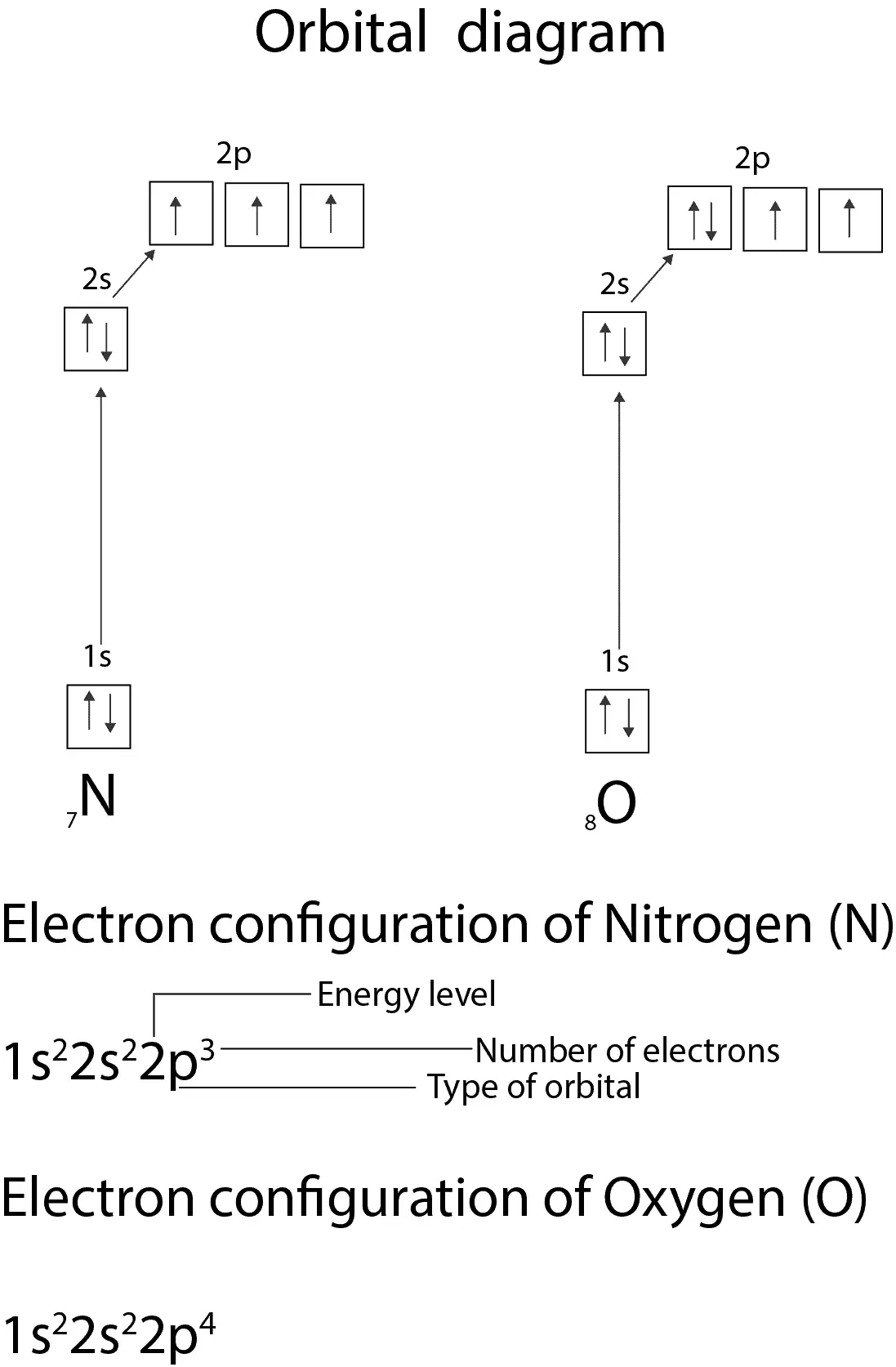
Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p . Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be.Given the same amount of absorbed solar energy coming in, the amount of IR escaping to space at the top of the ... Figure 3. Hybridization of an s orbital (blue) and a p orbital (red) of the same atom produces two sp hybrid orbitals (purple). Each hybrid orbital is oriented primarily in just one direction. Note that each sp orbital contains one lobe that is significantly larger than the other. The set of two sp orbitals are oriented at 180°, which is consistent with the geometry for two domains. The atomic number of nitrogen(N) is 7. The atomic number of an element is the number of electrons in that element. Therefore, the number of electrons in the nitrogen is seven. The main topic of this article is the nitrogen electron configuration and the orbital diagram.
Nitrogen atomic orbital diagram. Jun 13, 2016 · Write the molecular orbital diagram of N2+ and calculate their bond order why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric. Explain As we all know that nitrogen is an element that is a part of the periodic table. For those users who are not aware of the symbol of the element nitrogen, then it is represented by “N”. Electron Configuration For Nitrogen Ion. The atomic number of nitrogen is 7, the element nitrogen was discovered by a Scottish physician, Danial Rutherford. Create the atomic orbital diagram for nitrogen. Start by adding the appropriate subshells. For example, boron is in the 2p block of the periodic table, and so you need to show the 2p subshell and everything below it. Next, click the orbitals to add electrons (represented as arrows). For boron, you would need to show a total of five electrons. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the Aufau Principle to order the orbitals and hence the boxes, lines or circles, as shown below) Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen. Atomic orbital diagram for nitrogen. And three empty antibonding orbitals. The remaining three electrons will go in the 2p orbital. Molecular orbital energy level diagram of nitrogenoxygen duration. Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals. Here is a schematic orbital diagram for a hydrogen ... 2s. 20.3. N. 2p. 14.5. Transformation of the valence atomic orbitals in C3v symmetry. The following table summarizes how the nitrogen and hydrogen atomic orbitals transform. Additional discussion follows regarding the assignment of characters for the 2px and 2py orbitals which transform together as a pair. C 3v.
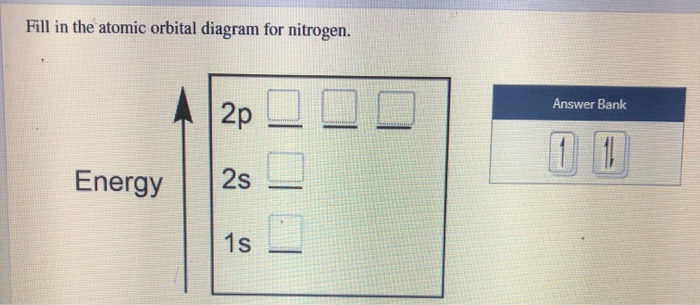
Nitrogen is the chemical element with the symbol N and atomic number 7. It was first discovered and isolated by Scottish physician Daniel Rutherford in 1772. Although Carl Wilhelm Scheele and Henry Cavendish had independently done so at about the same time, Rutherford is generally accorded the credit because his work was published first. The name nitrogène was suggested by French chemist … To see this video, other videos, chemistry education text, and practice problems visit my website. Website is 100% FREE to use.http://scientifictutor.org/ Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for N goes in the 2s orbital. The remaining three electrons will go in the 2p orbital. Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (17 ratings) Transcribed image text: Fill in the atomic orbital diagram for nitrogen. Answer Bank Energy Construct the orbital diagram for nickel. 1000 Answer Bank Energy 2 _ _ _.

Orbital Diagram Vs Electron Configuration Orbital Diagram Of Nitrogen Hd Png Download Transparent Png Image Pngitem
The atomic number of nitrogen(N) is 7. The atomic number of an element is the number of electrons in that element. Therefore, the number of electrons in the nitrogen is seven. The main topic of this article is the nitrogen electron configuration and the orbital diagram.
Figure 3. Hybridization of an s orbital (blue) and a p orbital (red) of the same atom produces two sp hybrid orbitals (purple). Each hybrid orbital is oriented primarily in just one direction. Note that each sp orbital contains one lobe that is significantly larger than the other. The set of two sp orbitals are oriented at 180°, which is consistent with the geometry for two domains.
Diagram of Hund's rule in boron, carbon, nitrogen, and oxygen. Figure 1. The 2p . Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be.Given the same amount of absorbed solar energy coming in, the amount of IR escaping to space at the top of the ...
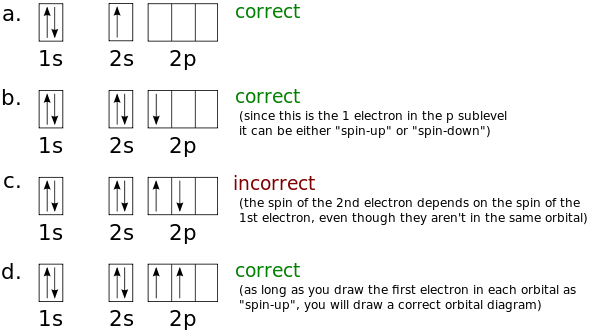
Solved A State Hund S Rule In Your Own Words And Show Its Application In The Orbital Diagram Of The Nitrogen Atom B For Main Group Elements Ar Course Hero

Above Orbital Diagram Shows The Electron Configuration Of Nitrogen Atom Which Rule Does Not Support This

Give Orbital Diagram Of The Following A Magnesium Chloride B Nitrogen C Methane D Hydrogen Chloride Flash Education

1 Create The Atomic Orbital Of Diagram For Nitrogen 2 Construct The Orbital Diagram For Ni Homeworklib
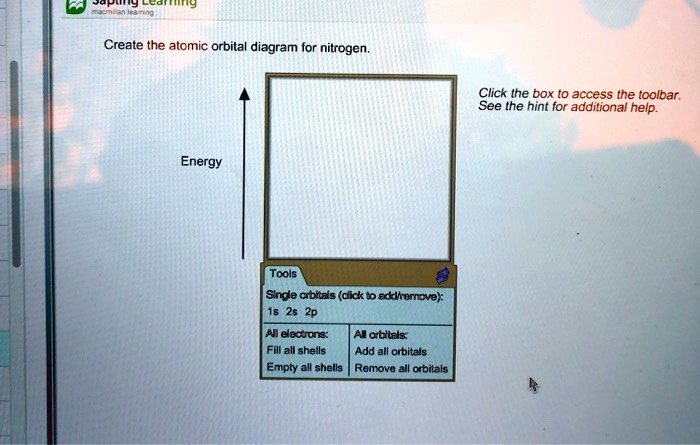
Solved Create The Atomic Orbital Diagram For Nitrogen Click Ihe Box T0 Access The Toolbar See The Hint For Additional Help Energy Tools Snde Arbials Dick T0 Edtmromove 26 2p Eectunz Odtel Fill

Struktur Lewis Orbital Atom Diagram Orbital Molekul Hibridisasi Orbital Rantai Molekul Bermacam Macam Sudut Png Pngegg


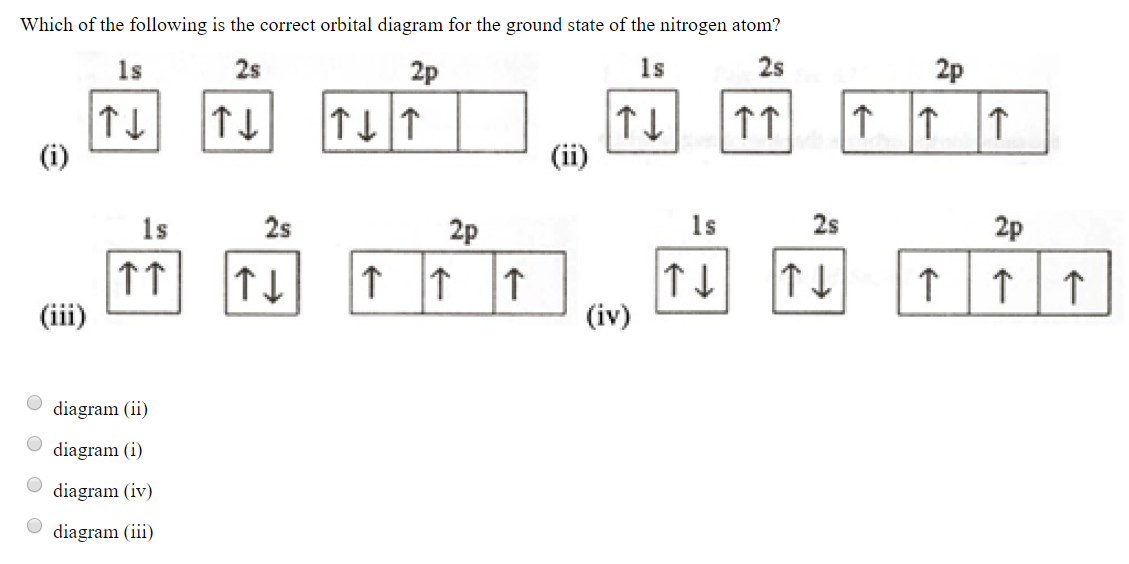

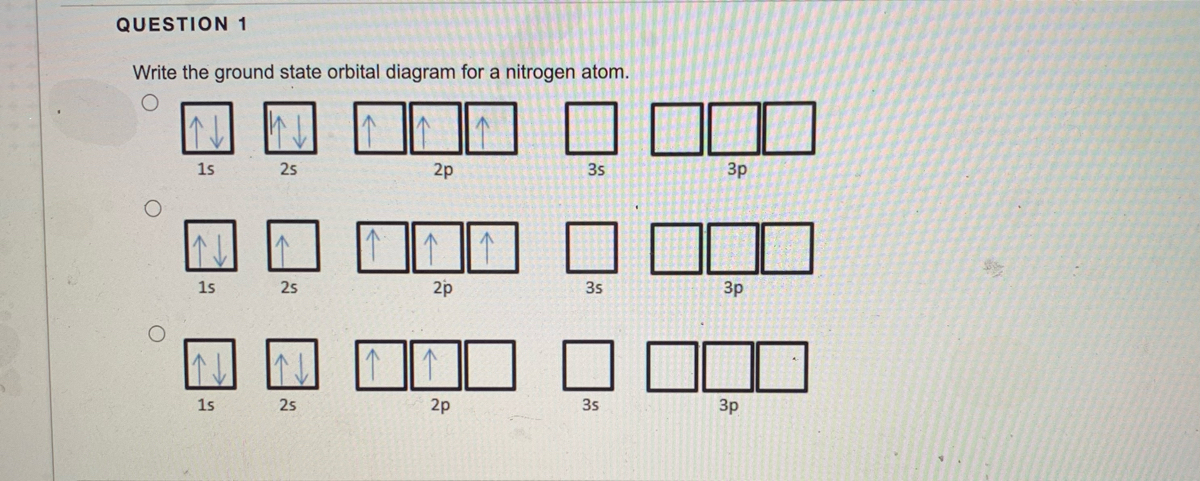

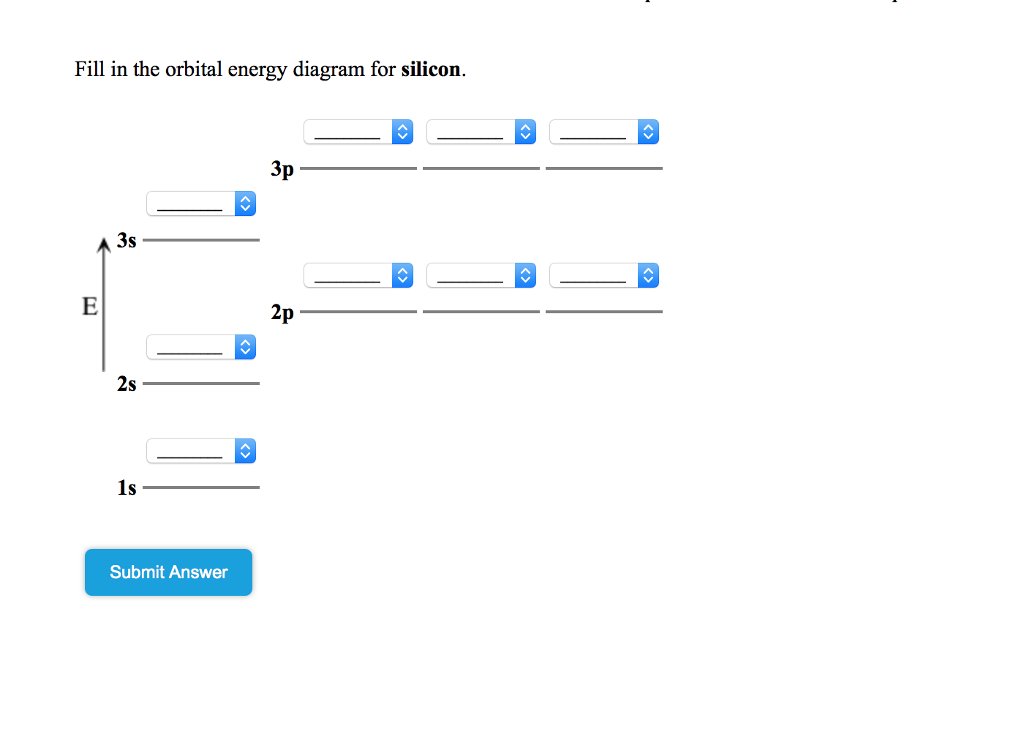
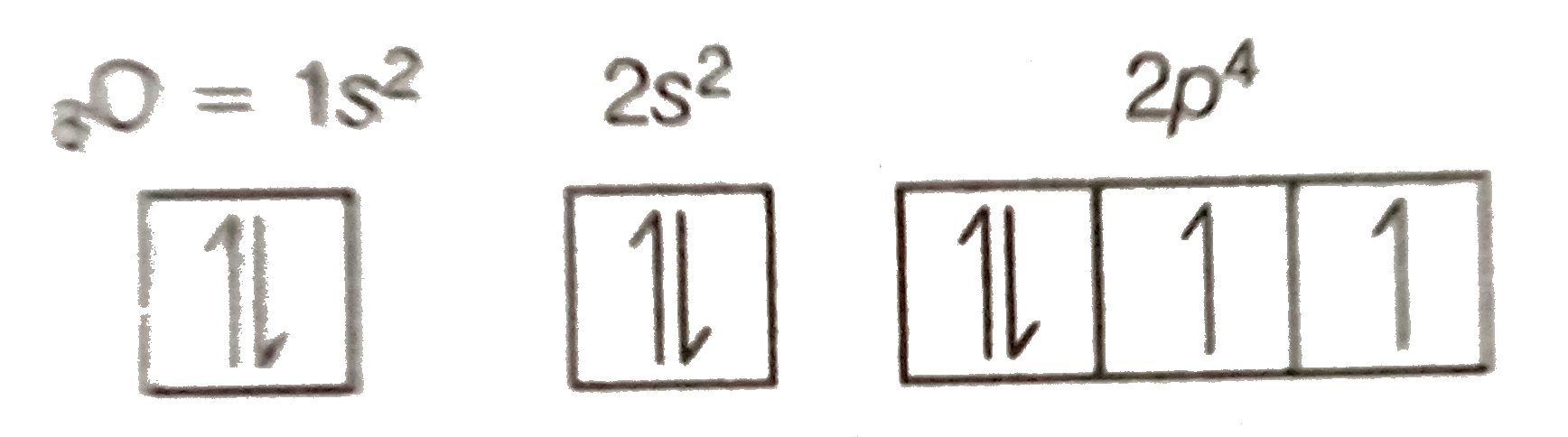






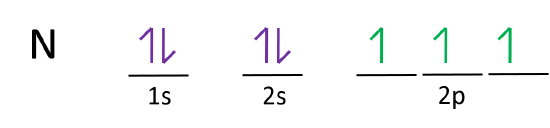







0 Response to "42 nitrogen atomic orbital diagram"
Post a Comment