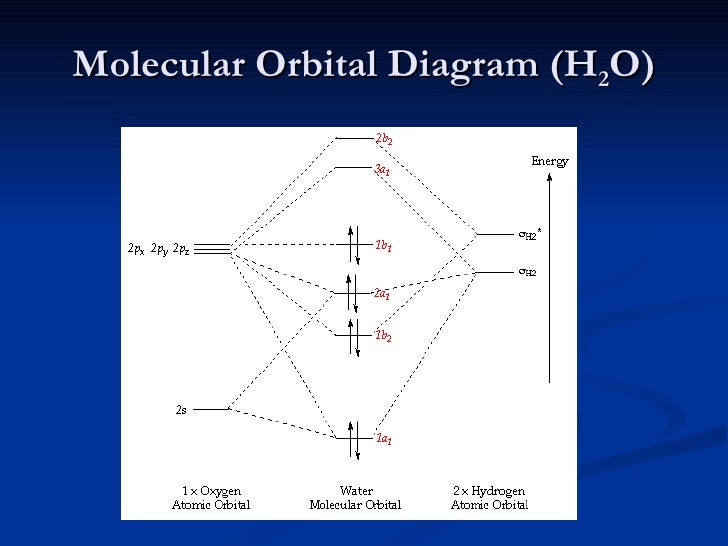
43 h2o molecular orbital diagram
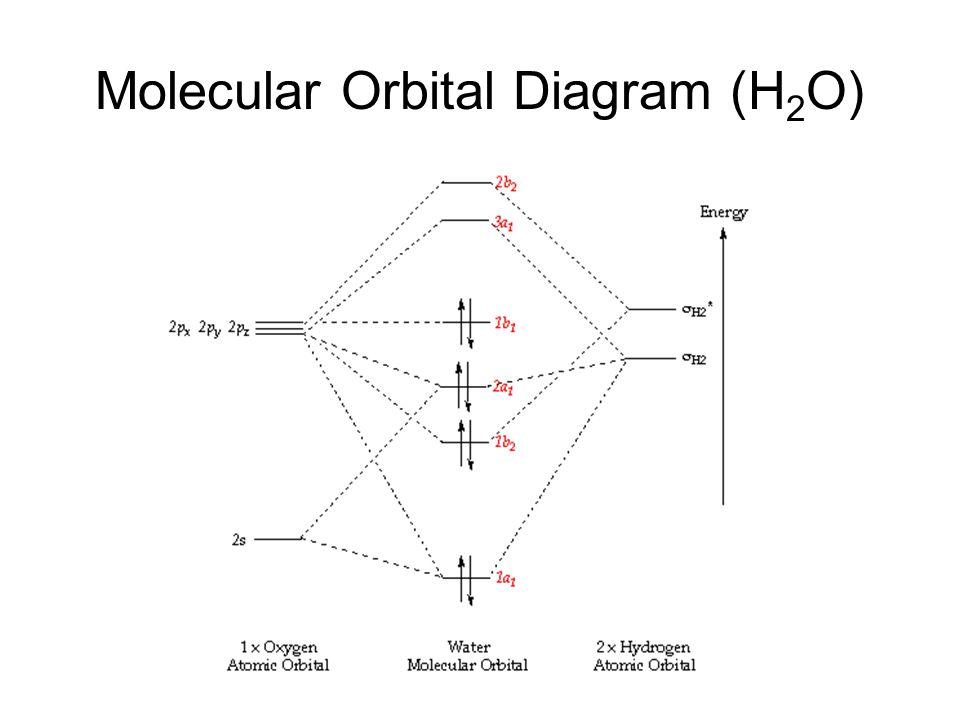
A molecular orbital diagram showing both the bonding and anti-bonding. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals. Generate the Molecular Orbitals for CH4(Td), CH4(D4h) and Cyclopropane using diagram between the bonding MOs of ... Construct SALCs and the molecular orbital diagram for H\(_2\)O. This is the first example so far that is not a linear molecule. Water is a bent molecule, and so it is important to remeber that interactions of pendant ligands are dependent on their position in space.
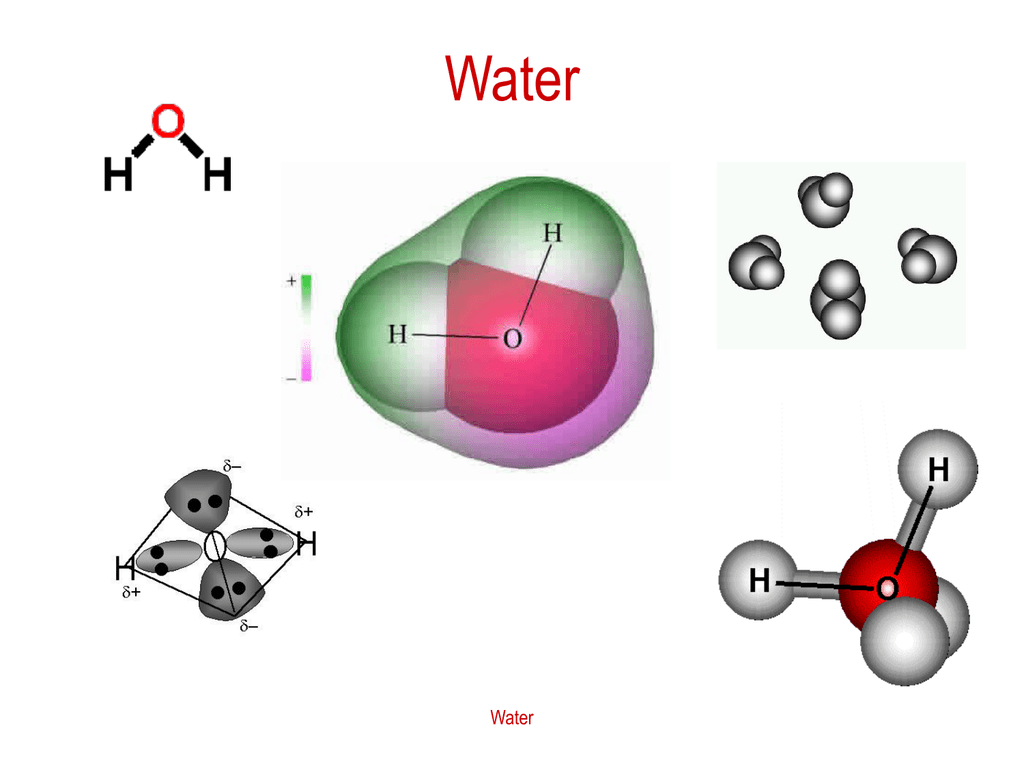
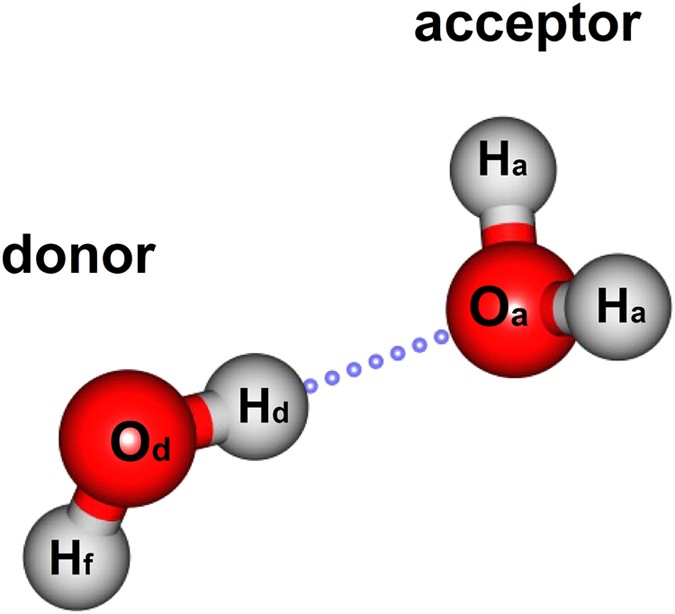
Water (H 2 O) is a simple triatomic bent molecule with C 2v molecular symmetry and bond angle of 104.5° between the central oxygen atom and the hydrogen atoms. Despite being one of the simplest triatomic molecules, its chemical bonding scheme is nonetheless complex as many of its bonding properties such as bond angle, ionization energy, and electronic state energy cannot be explained by one ...
H2o molecular orbital diagram
An advanced molecular orbital diagram of H2O (water) for the inorganic or physical chemistry student. sp 2 Hybridization. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry (). parallel overlap of the two unhybridized p atomic orbitals from each carbon. 15. Ethane, C2H6, has 2(4) + 6(1) = 14 valence electrons. The Lewis structure is: The carbon atoms are sp3 hybridized. The six C‒H sigma bonds are formed from overlap of the sp3 hybrid orbitals on C with the 1s atomic orbitals from the hydrogen atoms. The carbon-
H2o molecular orbital diagram. Three 2p orbitals of Oxygen and one 2s orbital are hybridized as there are two pairs of bonding electrons and two lone pairs. And as four orbitals of Oxygen are hybridized, the hybridization of H 2 O is sp3. H2O Molecular Geometry. The molecular geometry of any molecule depends on its Lewis structure, the arrangement of atoms and its electrons. Molecular Orbital diagram of water (H2O) The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound. Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure. Description. H2O-MO-Diagram.svg. English: MO diagram of water. Vectorized, simplified and corrected from File:Diagramme AH2.png. Quantitative calculations show bonding character in both the 3a 1 and 2a 1 levels. Date. 21 May 2015. Source. Own work. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
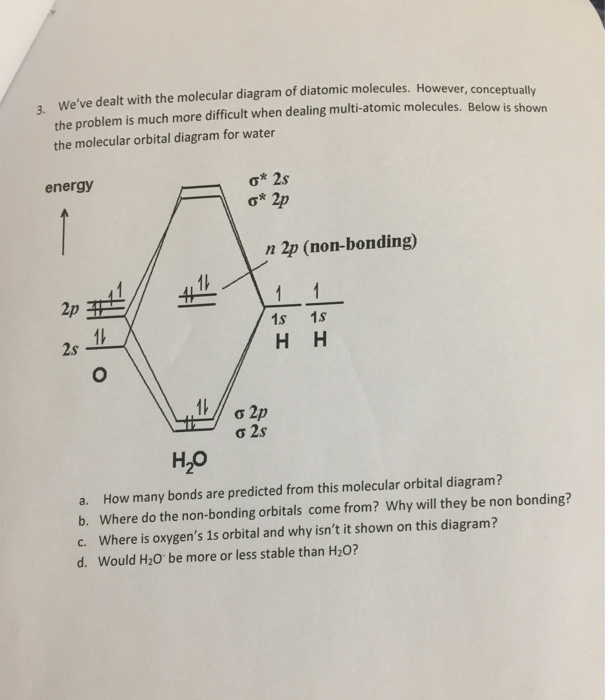
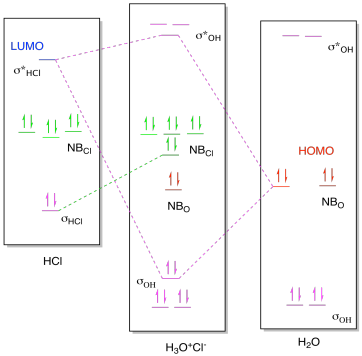
Question: 6. Consider the molecular orbital diagram for H20 and respond to the following questions: (a) (1) What is the total number of the formally bonding and antibonding orbitals in H2O? Answer: (b) (1) List all oxygen orbitals involved in bonding in H20. Answer: (c) (2) Name the oxygen orbitals that would not have been involved in bonding ... Here, using density functional theory and post-Hartree-Fock methods, we reveal two hydrogen bonding molecular orbitals crossing the hydrogen-bond's O and H atoms in the water dimer. Energy ... Molecular Orbitals for Larger Molecules 1. Determine point group of molecule (if linear, use D2h and C2v instead of D∞h or C∞v) 2. Assign x, y, z coordinates (z axis is principal axis; if non-linear, y axes of outer atoms point to central atom)3. Find the characters of the reducible representationfor the combination of Hybradization of the orbitals to [tex]sp^3[/tex] would darastically decrease the repulsion the previous model produces. so the hydrogens would bond to the two avaliable sp3 orbitals. As for the molecular orbital diagram, the lowest energy bonding interaction is a sigma interaction between hydrogen and the afore mentioned hybridization.
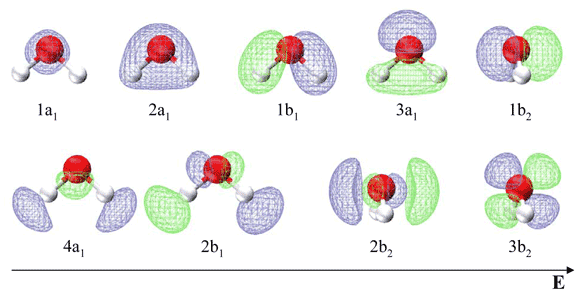
molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104 ... The MO Diagram for Water. Revision. • molecular orbitals are combinations of atomic orbitals. • atomic orbitals: o atomic orbitals have a radial and angular. Molecular orbital diagram of H2O Chemistry Titration/Moles Multiple Choice Question [PLEASE CHECK] Group 2 elements electrocell problem Dative covalent bonding - H2F+ Hydrolysis of polyesters MO diagram of water : why does the 2s interact ? Explain how oxidation will affect the bond length of F2. ... Molecular Orbitals for Water (H 2 O) The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2(2a 1) 2(1b 2) 2(3a 1) 2(1b 1) 2 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set (experimental data is given in [1289]). They are set out with the lowest
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Chemical bonding of H2O - WikipediaChemical bonding of H2O - Wikipedia
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
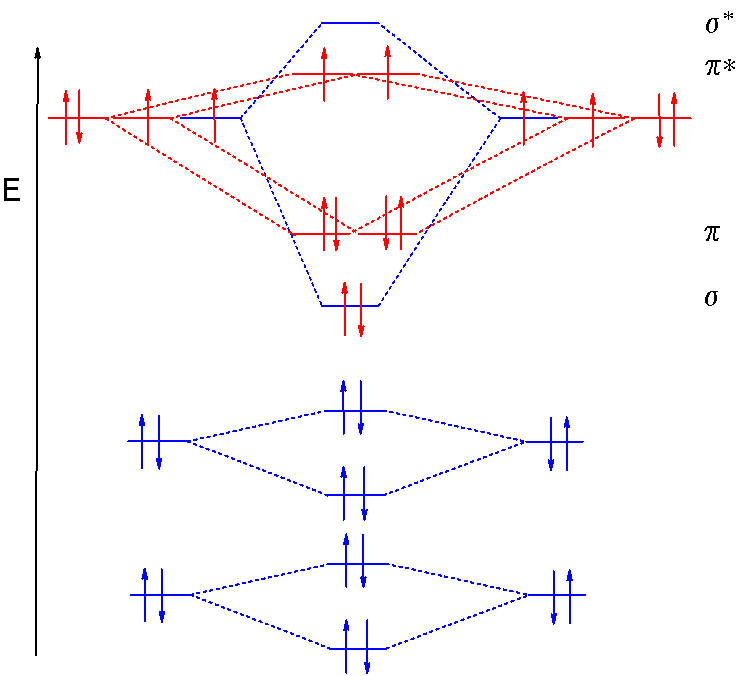
The atomic orbitals combine to produce the following molecular orbital diagram: Here the 2 p g orbital is occupied by two electrons to give a total bond order of three. This corresponds well with the Lewis structure ( ), although the orbital approach tells us that there is one s and two p .
Molecular orbitals (MOs) are approximated as combinations of atomic orbitals (AOs) ... General Features of MO Diagrams ... MO Diagram for the Water Molecule.
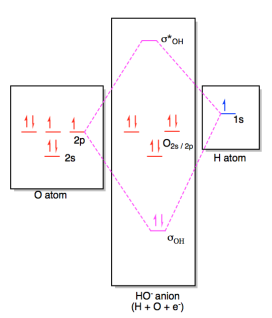
Energy level diagram for the molecular orbitals of OH ). H and O atom orbitals, which combine to form the molecular orbitals, are on the left and right side of the figure, respectively.
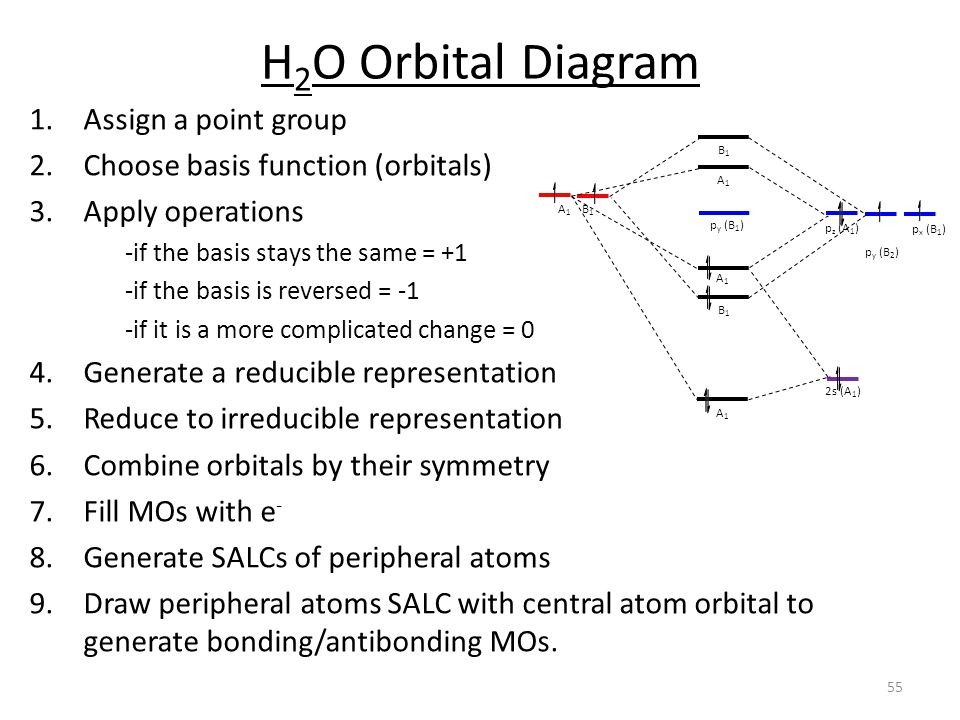
Using group theory to crate a qualitative MO diagram for water.
In general, this mixing of n atomic orbitals always generates n molecular orbitals. The hydrogen molecule provides a simple example of MO formation. In the following diagram, two 1s atomic orbitals combine to give a sigma (σ) bonding (low energy) molecular orbital and a second higher energy MO referred to as an antibonding orbital.
Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from o to 90 o Molecular Orbital .Molecular orbital diagram - WikipediaChemical bonding of H2O - Wikipedia
1. 199. Molecular Orbital Diagram of NO. TAGS. Molecular Orbital Diagram. Previous article Molecular Orbital Diagram of CO. Next article Qualitative and Quantitative Analysis |Organic Chemistry.
Draw a molecular orbital diagram for (a) H2, (b) H2O, (c) BHs, (d) CH4, and (e) [Fe(H)ol by performing the following steps i. Determine the point group of the molecule. Do not descend in symmetry ii. Determine the reducible representation (THIs) using the hydrogen 1s orbitals as your basis set. 111, Express the reducible representation as a ...
Follow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook page-https://lm.facebook.com/l.php?u=https%3A%2F%2Ffb.me%2F...
Molecular Orbitals for Water (H 2 O). The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2 (2a 1) 2 (1b 2) 2 (3a 1) 2 (1b 1) 2 (4a 1) 0 (2b 2) 0 (3b 2) 0 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. b (experimental data is given in []).They are set out with the lowest energy (that is, most ...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
parallel overlap of the two unhybridized p atomic orbitals from each carbon. 15. Ethane, C2H6, has 2(4) + 6(1) = 14 valence electrons. The Lewis structure is: The carbon atoms are sp3 hybridized. The six C‒H sigma bonds are formed from overlap of the sp3 hybrid orbitals on C with the 1s atomic orbitals from the hydrogen atoms. The carbon-
sp 2 Hybridization. The valence orbitals of a central atom surrounded by three regions of electron density consist of a set of three sp 2 hybrid orbitals and one unhybridized p orbital. This arrangement results from sp 2 hybridization, the mixing of one s orbital and two p orbitals to produce three identical hybrid orbitals oriented in a trigonal planar geometry ().
An advanced molecular orbital diagram of H2O (water) for the inorganic or physical chemistry student.




















0 Response to "43 h2o molecular orbital diagram"
Post a Comment