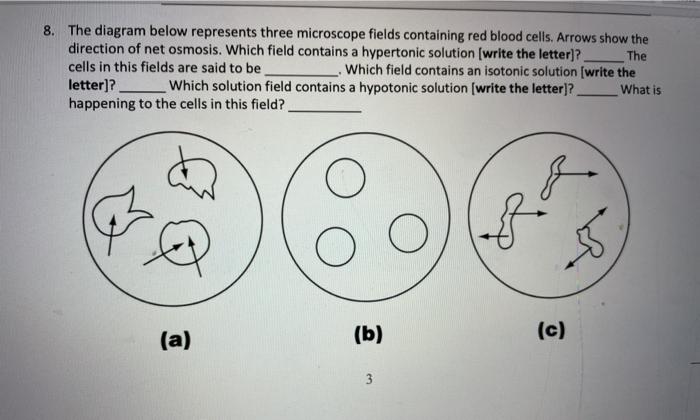
43 in the diagram which one represents a hypertonic solution
The diagram below represents part of alimentary canal. Study it and use it to answer the questions that follow. ... State one sample counting method which is not suitable in a densely forested habitat. (1 mark) ... /process by which solvent molecules move from hypotonic to hypertonic solution across semipermeable membrane; A plant cell when immersed in hypertonic solution like salt solution for about 30 minutes will become flaccid or limp. Question 4: Give reasons for the following: (a) If you sprinkle some common salt on grass growing on a lawn, it is killed at that spot.
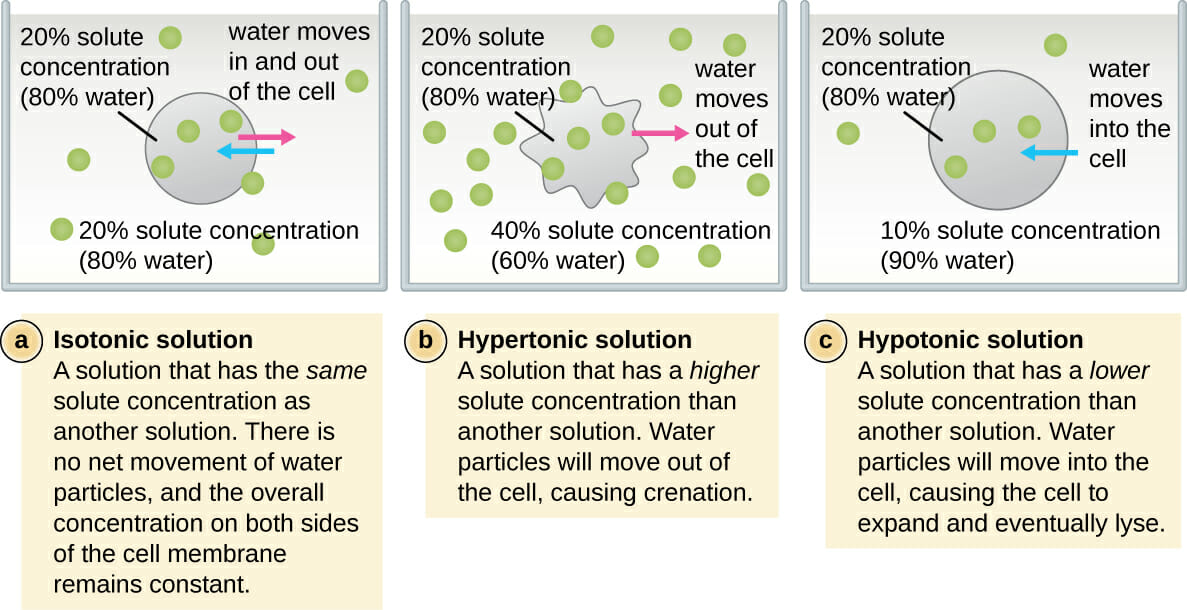
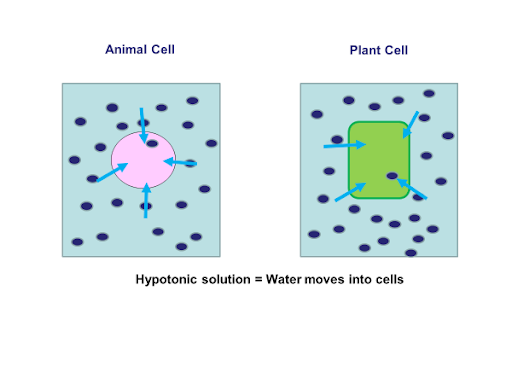
This is the process where solvent molecules (water) move from a lowly concentrated solution (dilute) to a highly concentrated solution across a semi-permeable membrane. Diagram fig 4.6. The highly concentrated solution is known as Hypertonic Solution. The lowly concentrated solution is called Hypotonic solution.
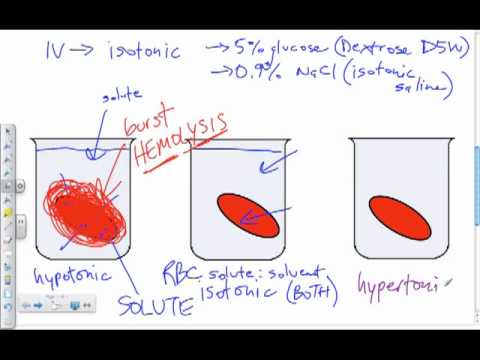
In the diagram which one represents a hypertonic solution
Answer: With regard to iodine, beaker is hypertonic whereas with regard to starch, tube is hypertonic. Explanation: The beaker has higher amount of iodine solution than the tube so the beaker is considered as hypertonic solution while on the other hand, the tube has more starch concentration than the beaker so the tube is considered as hypertonic solution. Question 1. Name the following: (a) The condition of a cell placed in a hypotonic solution. (b) Process by which intact plants lose water in the form of droplets from leaf margins. (c) Process by which water enters root hairs. (d) The tissue concerned with upward conduction of water in plants. (e) The term for the inward movement of solvent ... If π B is greater than π A, then the solution B is a hypertonic solution with respect to the solution A. Hence, if C A and C B are their concentrations, then C B > C A. Hence, for equal volume of the solutions, n B > n A. Question iii. A solvent and its solution containing a nonvolatile solute are separated by a semipermable membrane.
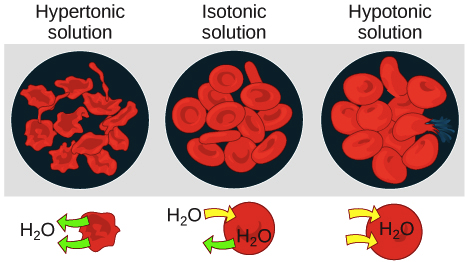
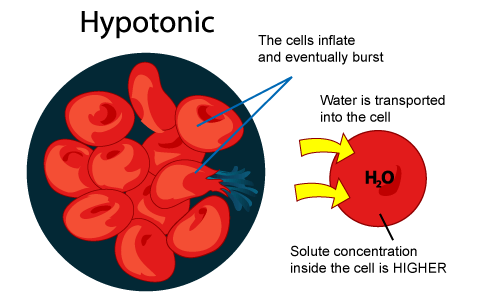
In the diagram which one represents a hypertonic solution. The diagram below represents a set up that was used to investigate a certain process in a plant. State the aim of the experiment.(1mk) State a factor that would affect the process.(1mk) State the importance of nucleic acids to an organisms.(1mk) State the significance of the following to a leaf:-Thinness(1mk) Presence of air spaces(1mk) What is Osmosis? By definition, osmosis is the movement of any solvent through a selectively permeable membrane into an area of higher solute concentration, the result of which will be an equalizing of solute concentration on either side of the membrane.. This equilibrium is important for the efficient and optimized function of cells; as mentioned before, balance is the preferred state in a ... Hypertonic Solution Definition. A hypertonic solution contains a higher concentration of solutes compared to another solution. The opposite solution with a lower concentration is known as the hypotonic solution.Scientists must describe cell contents compared to the environment. If a cell is placed in a hypertonic solution, the cell is considered hypotonic. It was found that the use of a hypertonic solution as a medium allowed the reduction in the initial water content in the plant tissue (osmotic dehydration) before drying, and, at the same time, increased the rate of weight movement during the drying process, which resulted in a higher value of the water diffusion coefficient calculated in ...
A hypertonic solution may be any solution that has a greater concentration of solutes outside of the cell, and one example of this is saline solutions used in medical care. Recall the osmlarity represents the variety of moles that particles per liter of solution, ... which way two solutions have the same variety of particles, or hyperosmotic which method one solution is more concentrated than the other, or hyposmotic which way that one solution is less focused than the other. ... When put in a hypertonic solution ... The ecosystem is the structural and functional unit of ecology where the living organisms interact with each other and the surrounding environment. In other words, an ecosystem is a chain of interaction between organisms and their environment. The term "Ecosystem" was first coined by A.G.Tansley, an English botanist, in 1935. 8. In cell cycle, DNA replication takes place in [1996, 2000] (a) G 1 phase (b) G 2 phase (c) Mitotic metaphase (d) S phase. Solution: (d) G 1 phase, also called Gap I phase is characterized by increase in cell size. In the S phase or synthetic phase DNA molecules replicate.
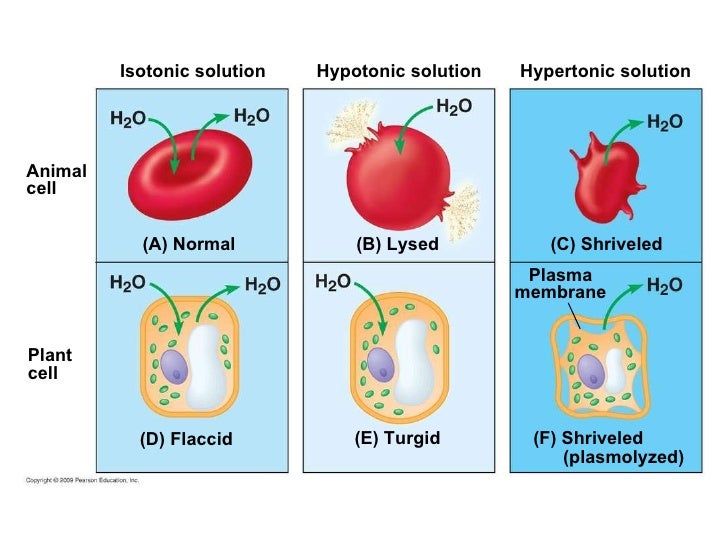
(ii) Hypertonic solution (iii) (1) Nucleus, (2) Sugar drops, (3) Small vacuole, (4) Large vacuole. (iv) Plant cell (1) Presence of cell wall, (2) Presence of large vacuole. (v) It has to be placed in a hypotonic solution. Question 4: The below diagram represents a plant cell after being placed in a strong sugar solution. Guidelines 1 to 5 ... 7.What will happen to a plant cell in a hypertonic solution? ... Name one other substance that moves into the cell by diffusion (1) Answer. Glucose (1) F. Answer. F. These substances pass through the cell membrane. Which letter represents the cell membrane in the diagram above. (1) Answer. B (1) Question 2. A student investigated the effect of ... Sketch a picture of one plant cell and label the cell wall, cell membrane, and chloroplasts. Next, remove the coverslip from the microscope slide, add a drop of 5% NaCl solution. Replace the coverslip and examine the slide under the microscope. Is the 5% NaCl solution isotonic, hypertonic, or hypotonic to the cell? (circle one) A hypotonic solution is one that has more water and fewer solutes than another solution. Explore the definition of a hypotonic solution and learn more through an example and diagram.
In The Diagram Which One Represents A Hypertonic Solution ... Medical and Health Science: Hypotonic, Isotonic ... Hypertonic solution causes cells to shrink and shrivel up ...
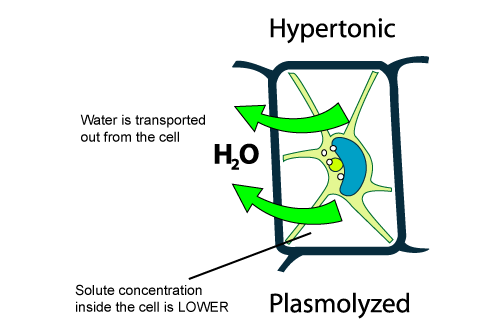
Solutions containing greater than 300 mOsm of nonpenetrating solutes (hypertonic solutions) cause cells to shrink as water diffuses out of the cell into the fluid with the lower water concentration. Note that the concentration of nonpenetrating solutes in a solution, not the total osmolarity, determines its tonicity — hypotonic , isotonic, or ...

Functional Significance Of The Cell Volume For Detecting Sperm Membrane Changes And Predicting Freezability In Dog Semen In Reproduction Volume 128 Issue 6 2004
In the diagram, which one represents facilitated diffusion? a. A b. B c. C d. Both a and c e. Both b and c Ans: E Difficulty: easy Feedback: 3.3 62. In the diagram, which one represents a hypertonic solution? a. A b. B c. C d. Both b and c e.
Dehydration is defined as the excessive loss of water from the body. The balance between fluid intake and fluid loss from the body is greatly disproportionate in dehydration. The severity of dehydration ranges from mild to severe, and dehydration can be fatal when fluid loss exceeds more than 15% of the total body water.
(c) 5%% sugar solution (d) Isotonic solution. Answer (d) Isotonic solution. Question 11 : Cell slightly enlarges or bursts when kept in (a) Hypertonic solution (b) Hypotonic solution (c) Isotonic solution (d) Pond water. Answer (b) Hypotonic solution. Question 12 : The diagram represents two liquids, separated by a membrane through which ...
(a) A plant cell, when placed in a hypotonic solution, receives water by osmosis. It does not burst because it is surrounded by a rigid cell wall which can withstand the turgor pressure of the turgid cell contents. <br> (b) When a fully turgid plant cell is placed in a hypertonic solution, the cytoplasm alongwith plasma membrane shrinks and separates from the cell wall as water flows out from ...
BIO201 Topic 3: Cell Biology Quiz September 2021 Question 1 The lower limit of resolution of a light microscope is Select one: a. 100μm b. 0.1μm c. 10μm d. 0.01μm e. 1.0μm Question 2 What structure does "C" represent on the diagram of the plasma membrane? Select one: a. membrane channel protein b. phospholipid bilayer c. internal membrane surface d. peripheral protein e. integral protein ...
in diagram, which one represents hypertonic solution a. A b. B c. C d. both B & C e. all choices are correct. ... red blood cells are in osmotic equilibrium when they are in a 0.9% NaCl solution. rapid osmotic efflux of water will occur if a cell is placed in a _____ NaCl solution. rapid osmotic influx of water will occur if a cell is placed in ...
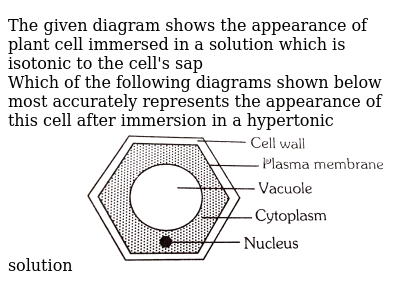
The Given Diagram Shows The Appearance Of Plant Cell Immersed In A Solution Which Is Isotonic To The Cell S Sap Which Of The Following Diagrams Shown Below Most Accurately Represents The Appearance
21. The diagram given below represents a plant cell after being placed in a strong sugar solution. Guidelines 1 to 5 indicate the following: 1. Strong sugar solution 2. Cell wall 3. Protoplasm 4. Large vacuole 5. Nucleus Study the diagram and answer the questions that follow: (i) What is the state of the cell shown in the diagram?

Pannexin 1 Constitutes The Large Conductance Cation Channel Of Cardiac Myocytes Journal Of Biological Chemistry
a. isotonic. b. hypotonicThis is the correct answer. =c. hypertonic. d. none of the above. b. hypotonic. In the diagram below, the solution on the right is considered _____________ becuase it has a higher concentration of solute (orange dots) distributed in the water. a. hypotonic. isotonic. c. none of the above.

Selina Solutions Concise Biology Class 10 Chapter 4 Absorption By Roots The Processes Involved Available In Free Pdf
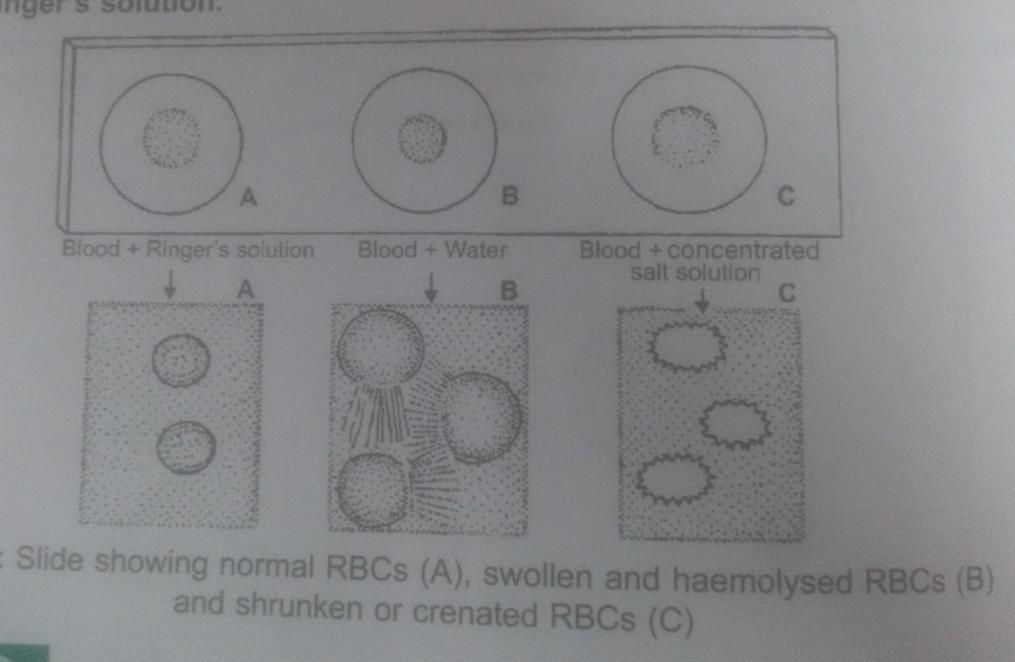
hypotonic solution is one in which the solution kept outside the cell has lower solute concentration than inside the cell. ... when a cell is kept in hypertonic solution. ... The diagram given below represents an experimental set-up to demonstrate a certain process. Study the same and answer the questions that follow:
Systemic And Renal Effects Of Preventing Contrast Nephrotoxicity With Isotonic 0 9 And Hypotonic 0 45 Saline Revista Espanola De Cardiologia
If π B is greater than π A, then the solution B is a hypertonic solution with respect to the solution A. Hence, if C A and C B are their concentrations, then C B > C A. Hence, for equal volume of the solutions, n B > n A. Question iii. A solvent and its solution containing a nonvolatile solute are separated by a semipermable membrane.

Effect Of Isotonic Versus Hypotonic Maintenance Fluid Therapy On Urine Output Fluid Balance And Electrolyte Homeostasis A Crossover Study In Fasting Adult Volunteers British Journal Of Anaesthesia
Question 1. Name the following: (a) The condition of a cell placed in a hypotonic solution. (b) Process by which intact plants lose water in the form of droplets from leaf margins. (c) Process by which water enters root hairs. (d) The tissue concerned with upward conduction of water in plants. (e) The term for the inward movement of solvent ...
Answer: With regard to iodine, beaker is hypertonic whereas with regard to starch, tube is hypertonic. Explanation: The beaker has higher amount of iodine solution than the tube so the beaker is considered as hypertonic solution while on the other hand, the tube has more starch concentration than the beaker so the tube is considered as hypertonic solution.

Treatment Patterns In People With Cystic Fibrosis Have They Changed Since The Introduction Of Ivacaftor Journal Of Cystic Fibrosis

Hypotonic Challenge Of Endothelial Cells Increases Membrane Stiffness With No Effect On Tether Force Sciencedirect

Role Playing Activity To Demonstrate Diffusion Across A Cell Membrane Journal Of Microbiology Biology Education
Plants Free Full Text Structure And Biomechanics During Xylem Vessel Transdifferentiation In Arabidopsis Thaliana Html
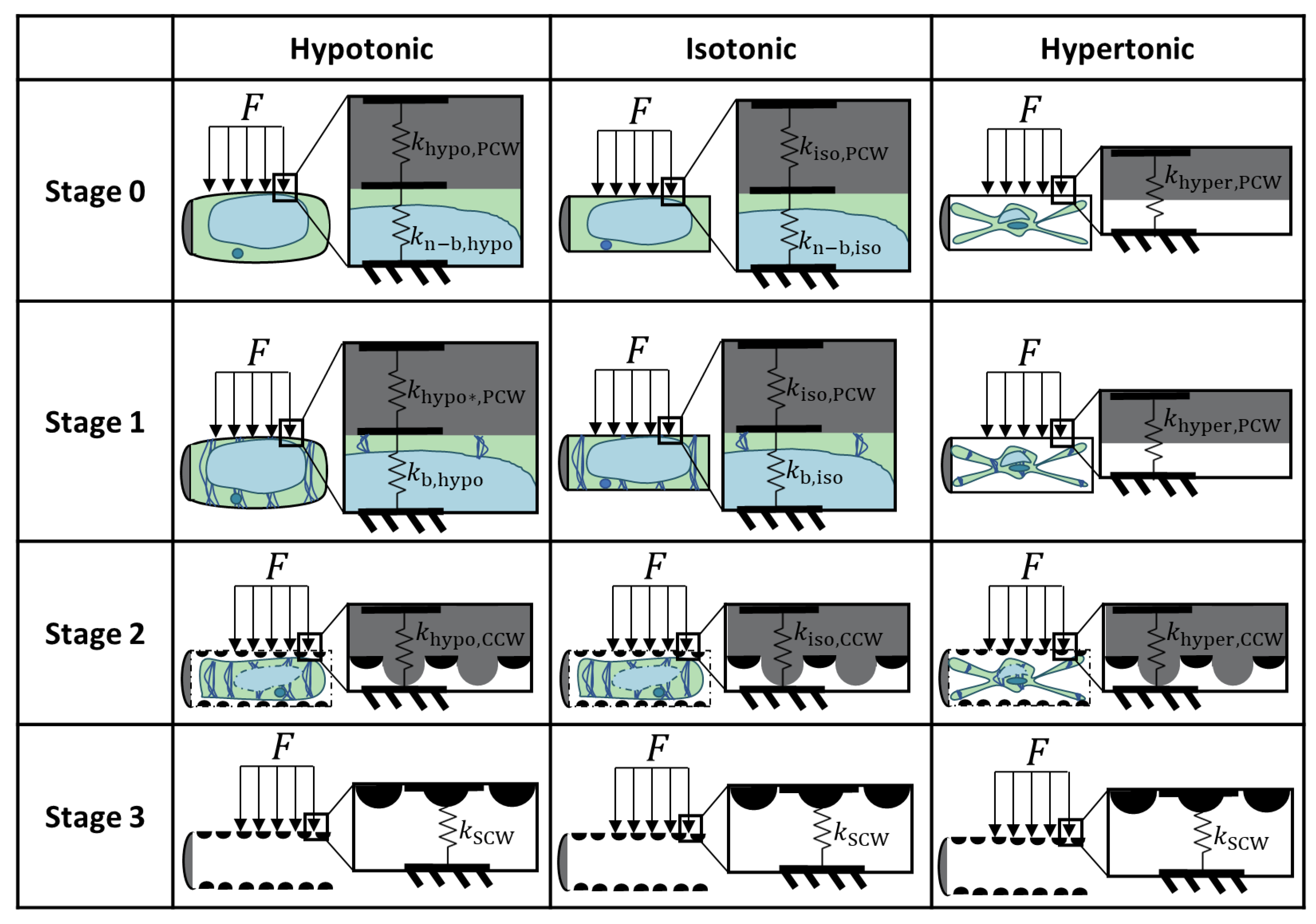
Plants Free Full Text Structure And Biomechanics During Xylem Vessel Transdifferentiation In Arabidopsis Thaliana Html
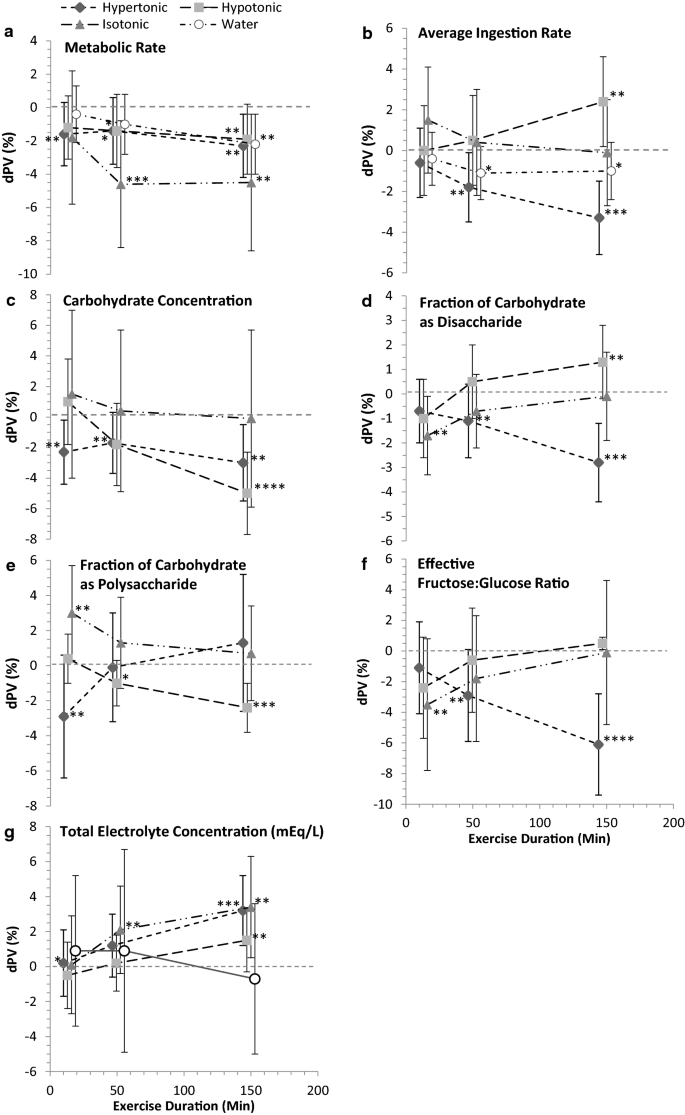
The Hydrating Effects Of Hypertonic Isotonic And Hypotonic Sports Drinks And Waters On Central Hydration During Continuous Exercise A Systematic Meta Analysis And Perspective Springerlink
Plos One Effect Of Intraoperative Hartmann S Versus Hypotonic Solution Administration On Flacc Pain Scale Scores In Children A Prospective Randomized Controlled Trial

Functional Significance Of The Cell Volume For Detecting Sperm Membrane Changes And Predicting Freezability In Dog Semen In Reproduction Volume 128 Issue 6 2004
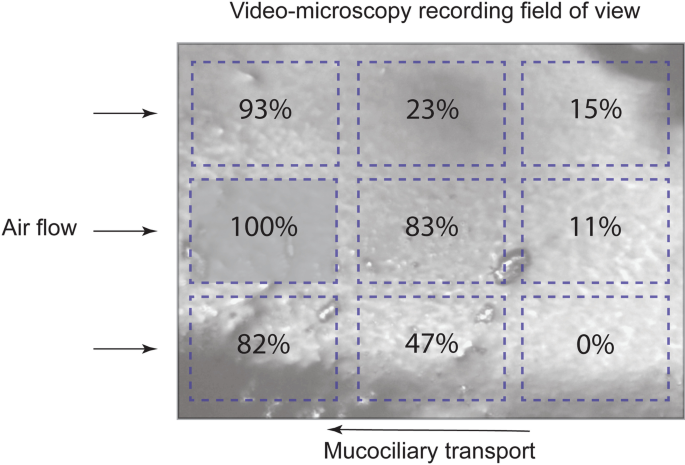
Rapid Changes In Mucociliary Transport In The Tracheal Epithelium Caused By Unconditioned Room Air Or Nebulized Hypertonic Saline And Mannitol Are Not Determined By Frequency Of Beating Cilia Intensive Care Medicine

The Given Diagram Shows The Appearance Of Plant Cell Immersed In A Solution Which Is Isotonic To The Cell S Sap Which Of The Following Diagrams Shown Below Most Accurately Represents The Appearance
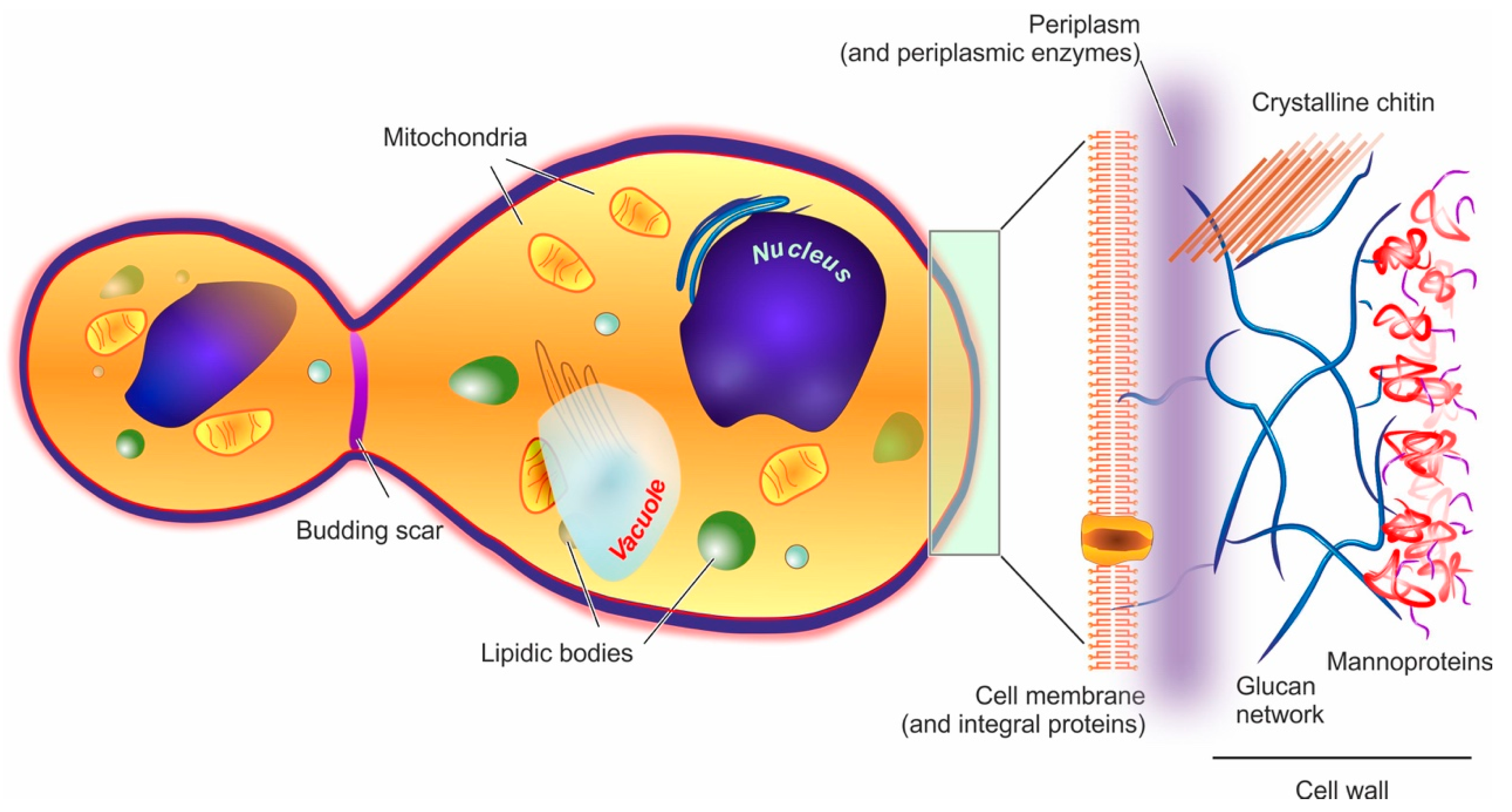
Molecules Free Full Text Yeast Cells In Microencapsulation General Features And Controlling Factors Of The Encapsulation Process Html

Amino Acids Are Compatible Osmolytes For Volume Recovery After Hypertonic Shrinkage In Vascular Endothelial Cells American Journal Of Physiology Cell Physiology

Examining The Effect Of Hypertonic Saline Administered For Reduction Of Intracranial Hypertension On Coagulation Journal Of The American College Of Surgeons













0 Response to "43 in the diagram which one represents a hypertonic solution"
Post a Comment