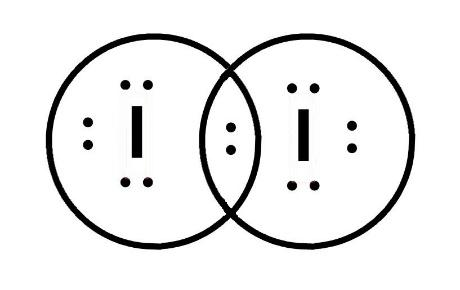
43 lewis dot diagram for iodine
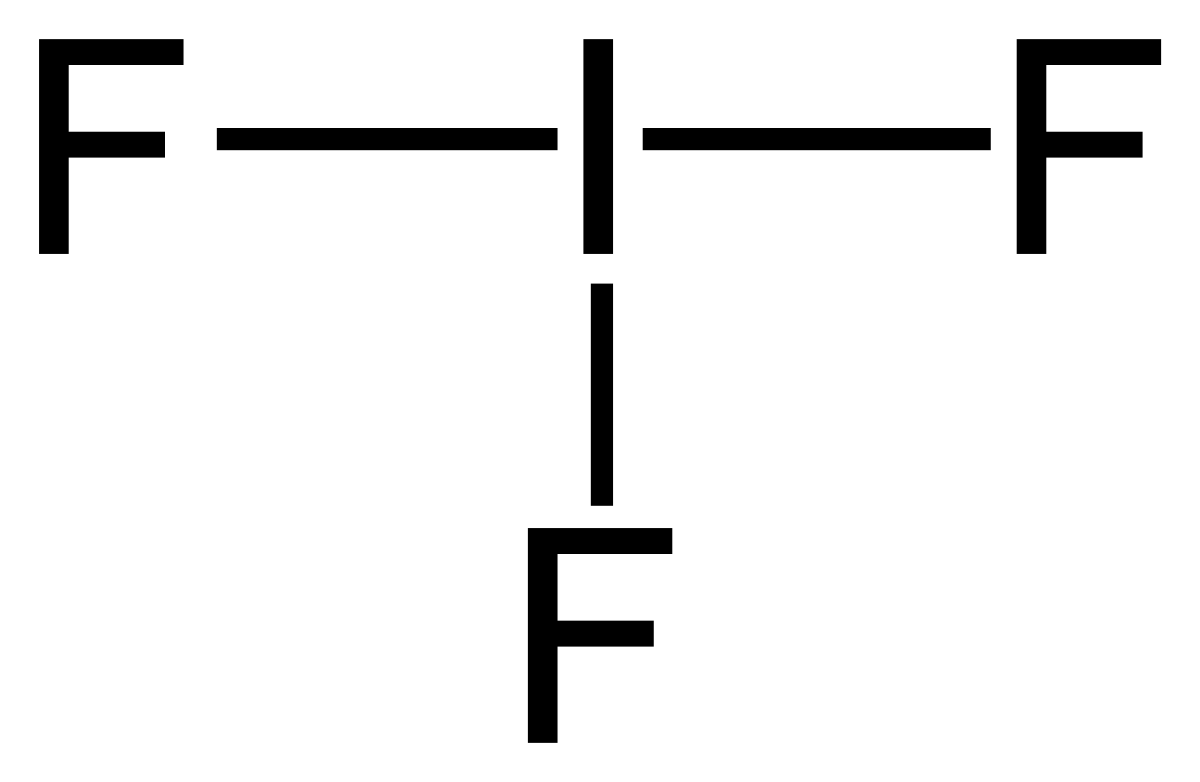
Atomic Structure of Iodine ... Electron Configuration: 1s2 2s2p6 3s2p6d10 4s2p6 ... For the following species, write Lewis dot structures, predict the molecular geometry, estimate all bond angles ... Around the iodine center there are 5 bonds and one lone pair, leading to an octahedral distribution of electron density. With one lone pair, the molecular shape becomes a square-based pyramid. All of the bond angles become slightly less than 90 o, perhaps about 89 o. The angles ...
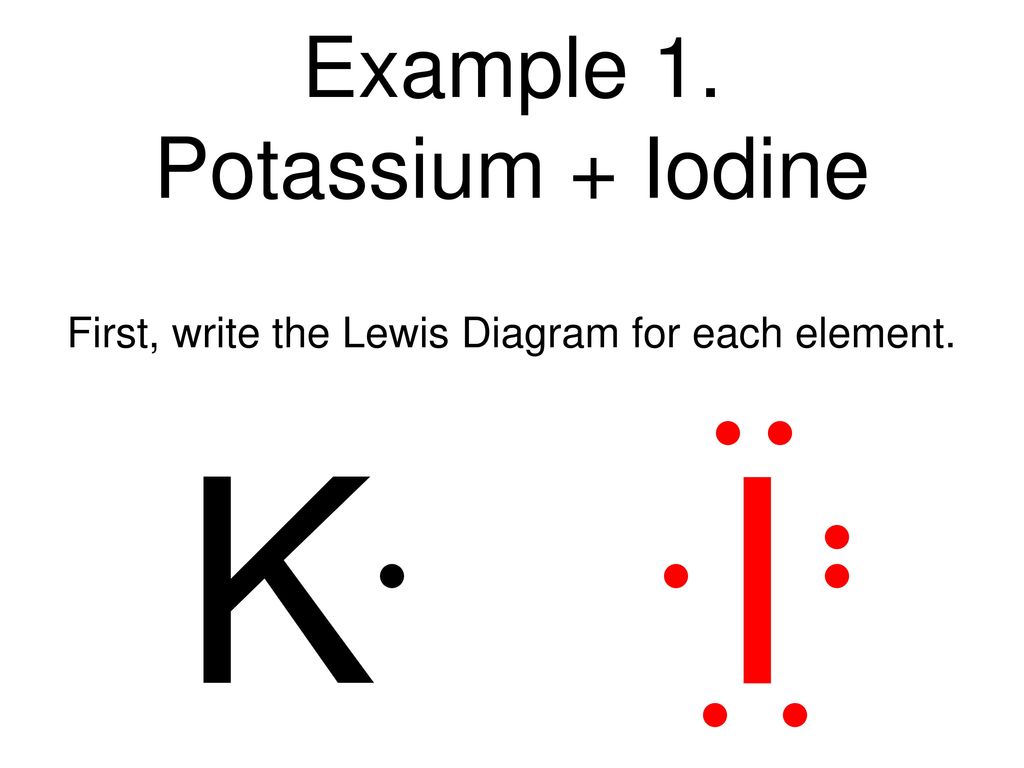
Dept 2252. (i) Complete the Lewis electron-dot diagram of the molecule in Box X. Problem 2. Chemistry and Physics. 423-425-4278. The LHS consists of the reactants and the RHS consists of the products. K a is the equilibrium constant for the dissociation reaction of a weak acid. If you are look for Ch3och2ch3 Structure, simply will check out our info below : Search by Systematic name, Synonym ...

Lewis dot diagram for iodine
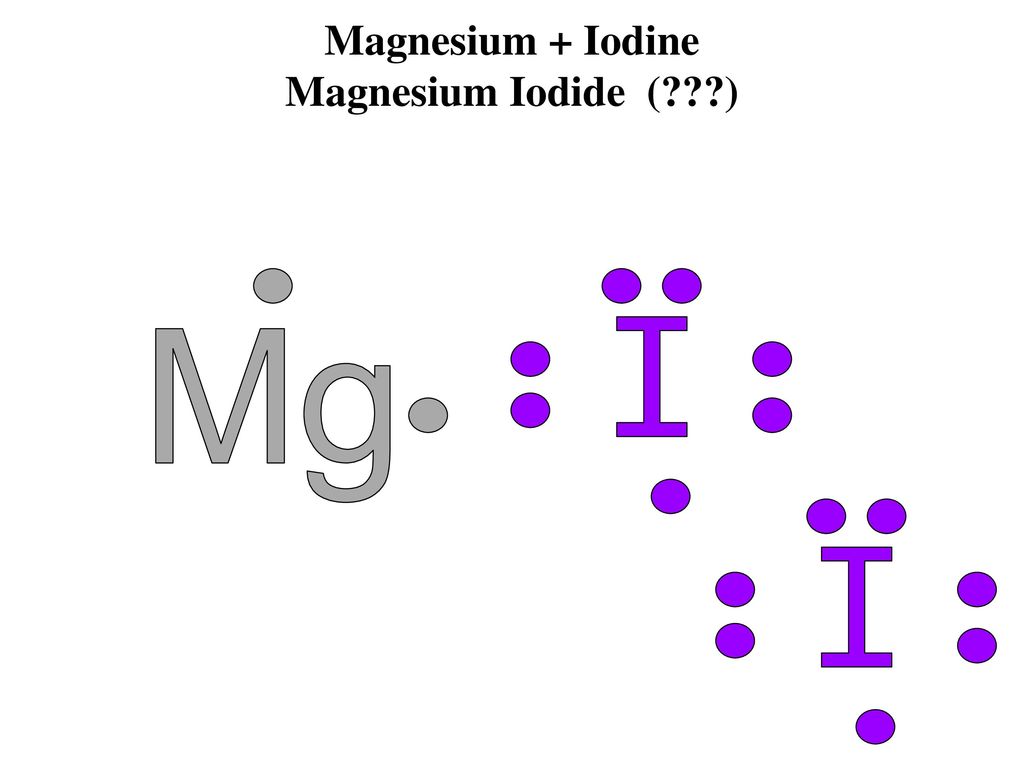
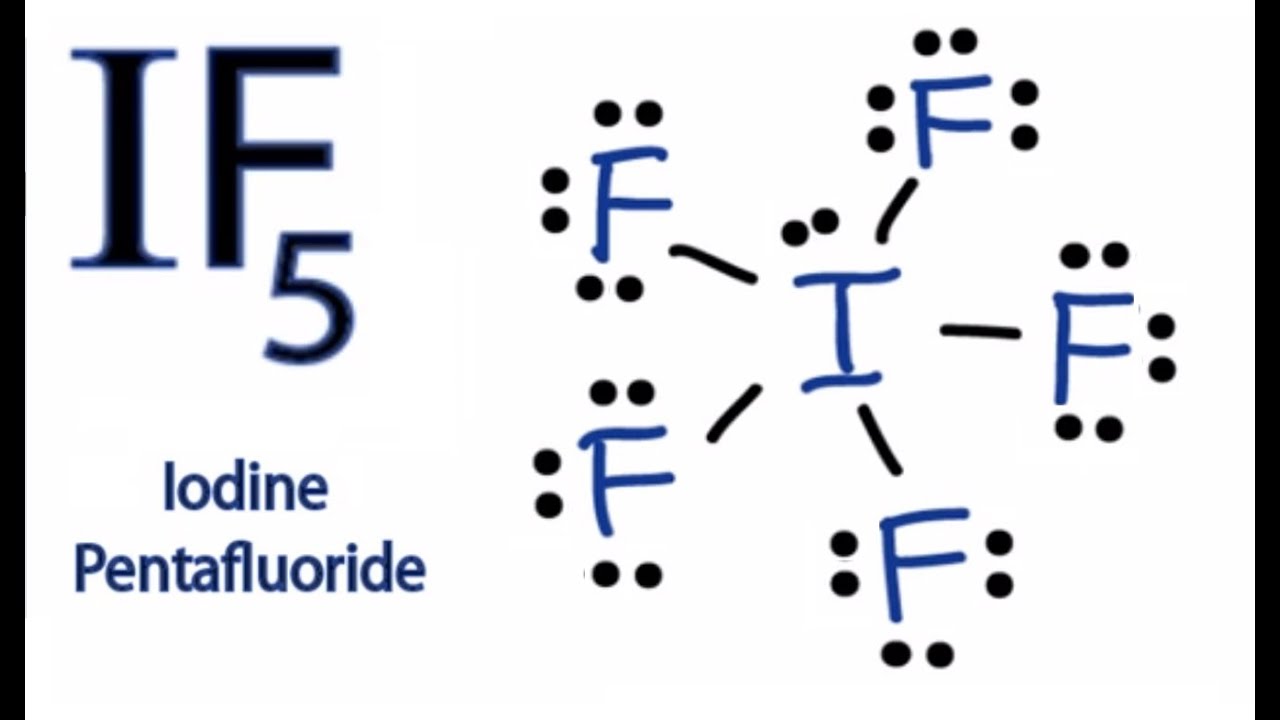
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct.A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis ... Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). In the Lewis structure for IF5 you' ...25 Oct 2016 · Uploaded by Wayne Breslyn 28 Apr 2021 — Iodine has seven valence electrons, because it is in group seven on the periodic table and is a halogen. The dot structure would be the element ...
Lewis dot diagram for iodine. 1 answerWhat should the electron dot diagram for iodine look like? Chemistry Drawing Lewis Structures. 1 Answer. Jahan Psyche. Oct 24, 2015. peoi.org. 2 days ago · For the AsH 3 Lewis structure there are a total of 8 valence electrons available. If you want to know about the lewis structure of BF3, you need to calculate the valence electrons for the BF3 molecule. > Draw the electron dot diagr A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. 8 8 8 OS O ... Lewis diagram for the ether c2h5oc2h5 27/10/2021 · Forming the single bonds with the other two iodine, we find out that there are 3 lone pairs and 2 bond pairs for the central iodine. 5. Checking the formal charge, we put the negative charge outside as per the diagram above. Thus, lewis structure is done. Hybridization of I3. The hybridization of I3 (Triiodide ion) is sp3d.
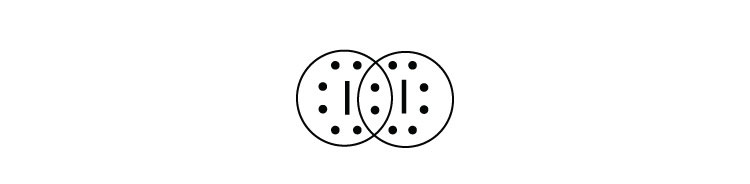
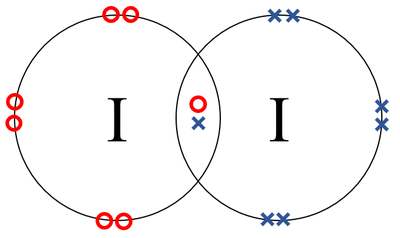
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. (It does ... (It does not matter what order the positions are used.) For example, the Lewis electron dot diagram for calcium is simply. A Lewis structure of calcium is shown ... ICl2- lewis structure contains one iodine atom at the middle position whereas two chlorine atoms at the surrounding position. There are three lone pairs present on the central atom of ICl2- lewis structure. Also, the iodine central atom in ICl2- lewis structure violates … 1 answerHint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, ...
The lewis dot diagram for carbon dioxide also shows that two pairs of electrons are shared. Introduction. While we talk related with ionic May 14, 2018 · Ionic bonding worksheet for each pair of elements below draw an atomic diagram showing electrons in different energy levels. Beside that, we also come with more related things as follows drawing ionic and covalent bonds worksheet, practice ... 15/11/2021 · Lewis Structure. To be very precise, Lewis Structure is the name given to the structural representation of a molecule. It is the diagrammatic layout for understanding the nitty-gritty of chemical bonding. A very essential concept of molecular chemistry, the following steps dictate how you can successfully draw Lewis Structure: Step 1. The initial step towards forming this structure is to find ... 28 Apr 2021 — Iodine has seven valence electrons, because it is in group seven on the periodic table and is a halogen. The dot structure would be the element ... Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). In the Lewis structure for IF5 you' ...25 Oct 2016 · Uploaded by Wayne Breslyn
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct.A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis ...

The Anion I42 Is Linear The Anion I5 Is Bent With A 95 Degree Angle At The Central Iodine Atom Draw Valid Lewis Structures For Each Of These Ions What Orbital Hybridizations Are

Yodium Pentafluoride Struktur Lewis Iodine Heptafluoride Arsenic Pentafluoride Lainnya Sudut Teks Lain Lain Png Pngwing
Solved Write The Correct Lewis Structure For Iodine Pentafluoride If5 And Identify The Electronic And Molecular Geometries Course Hero



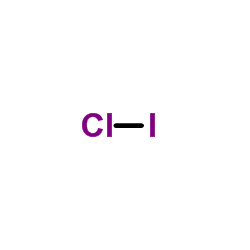






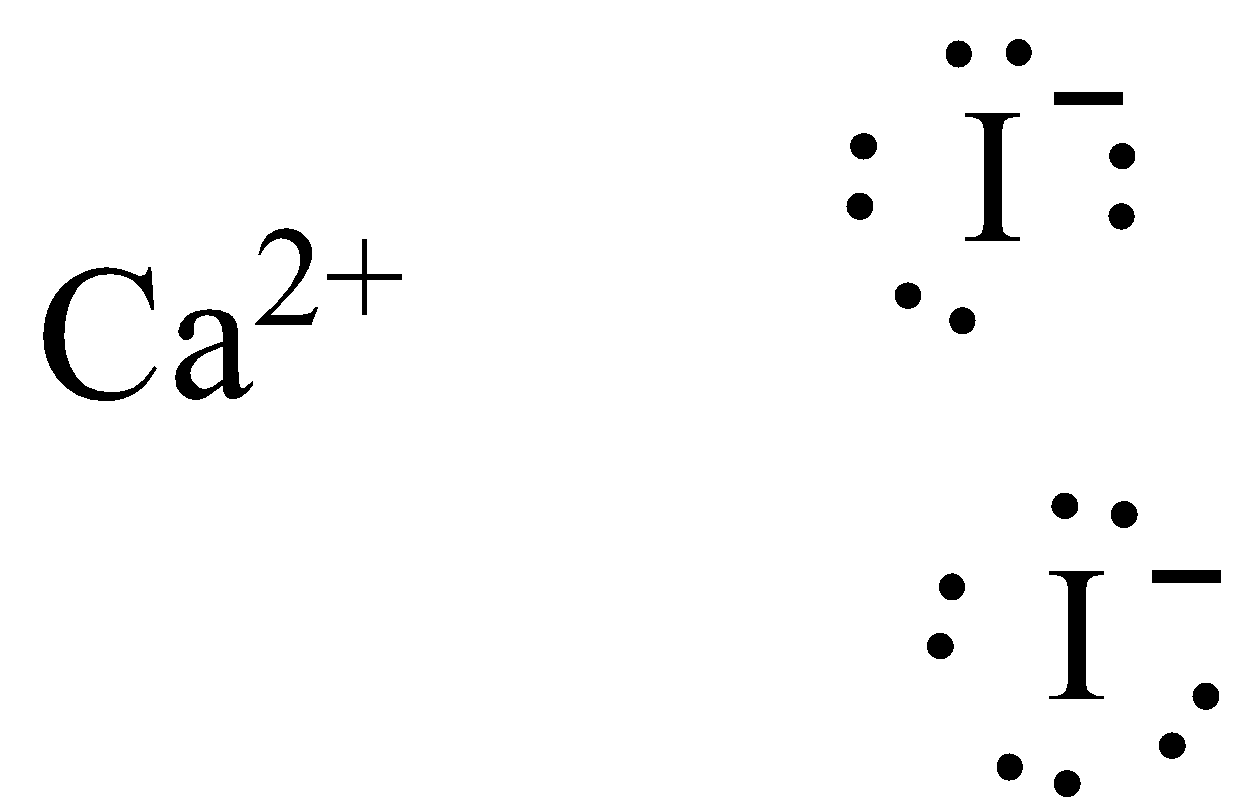













0 Response to "43 lewis dot diagram for iodine"
Post a Comment