43 tin electron dot diagram
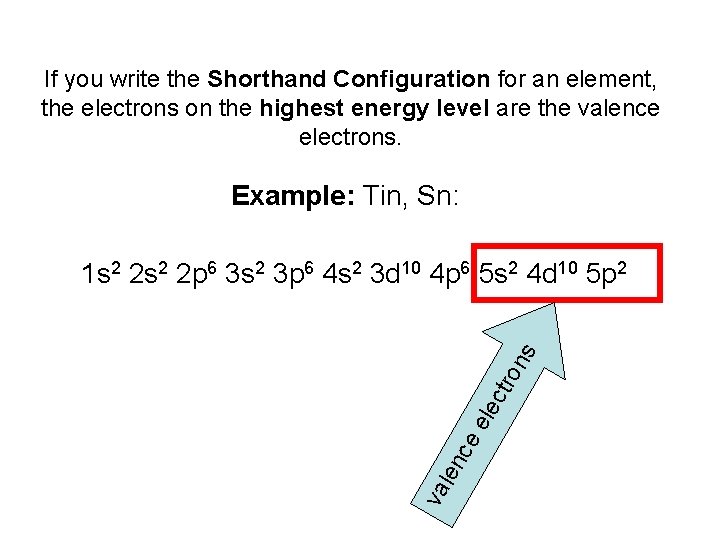
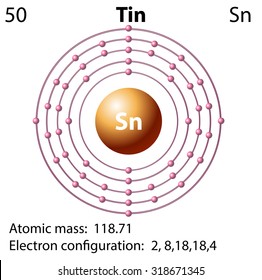
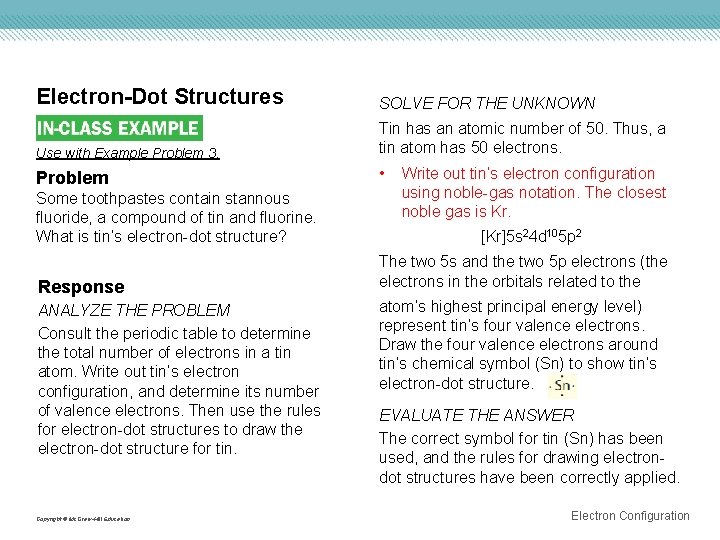
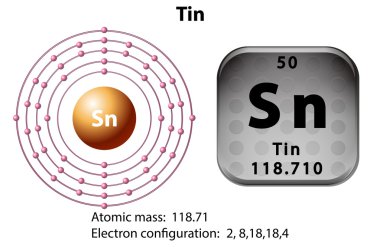
27 Jun 2016 · 1 answerTin has 4 valence electrons. Explanation: The quick answer here is that because tin, Sn , is a main-group element, the number of valance ... Tin (Sn), Electron Configuration: [Kr] 4d 10 5s 2 5p 2 [Kr] 4d 10 5s 2 5p 2::: example of: Concise Form of Electron Configuration Notation. Concise Form of Electron ...
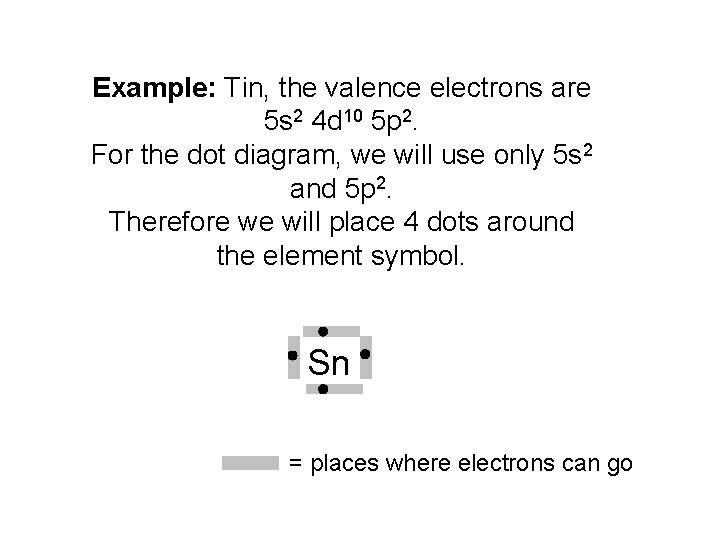
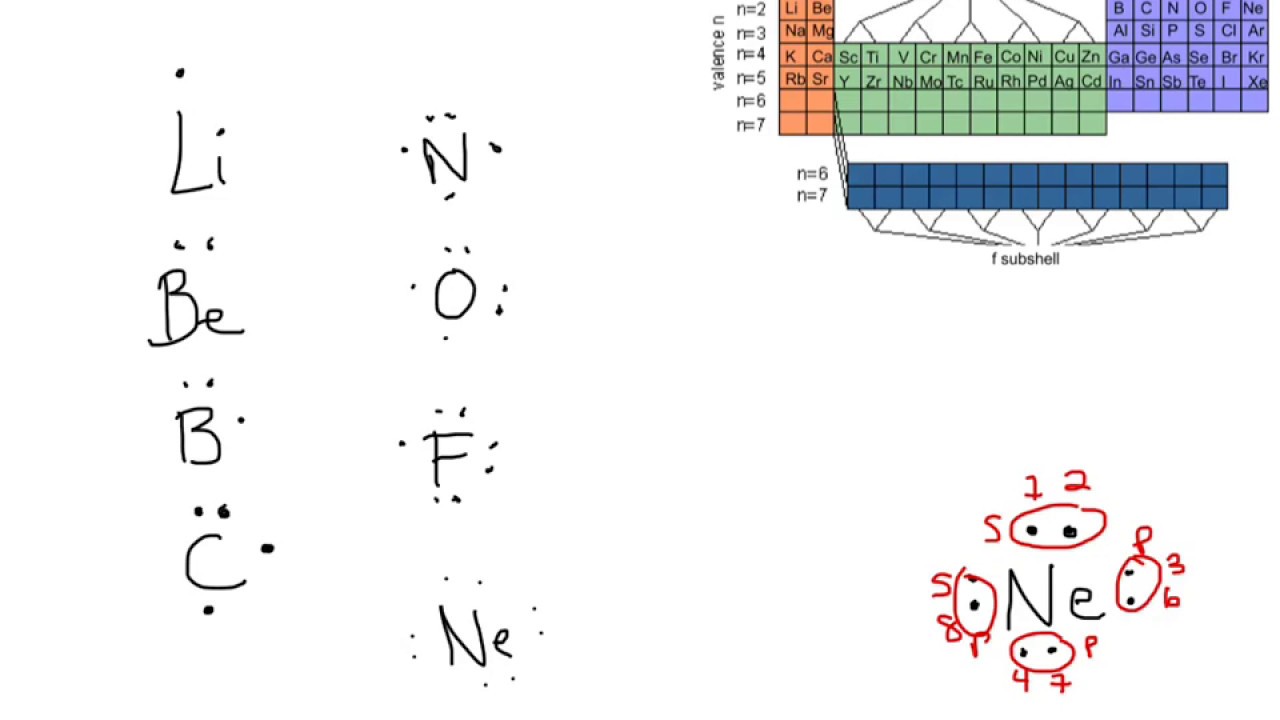
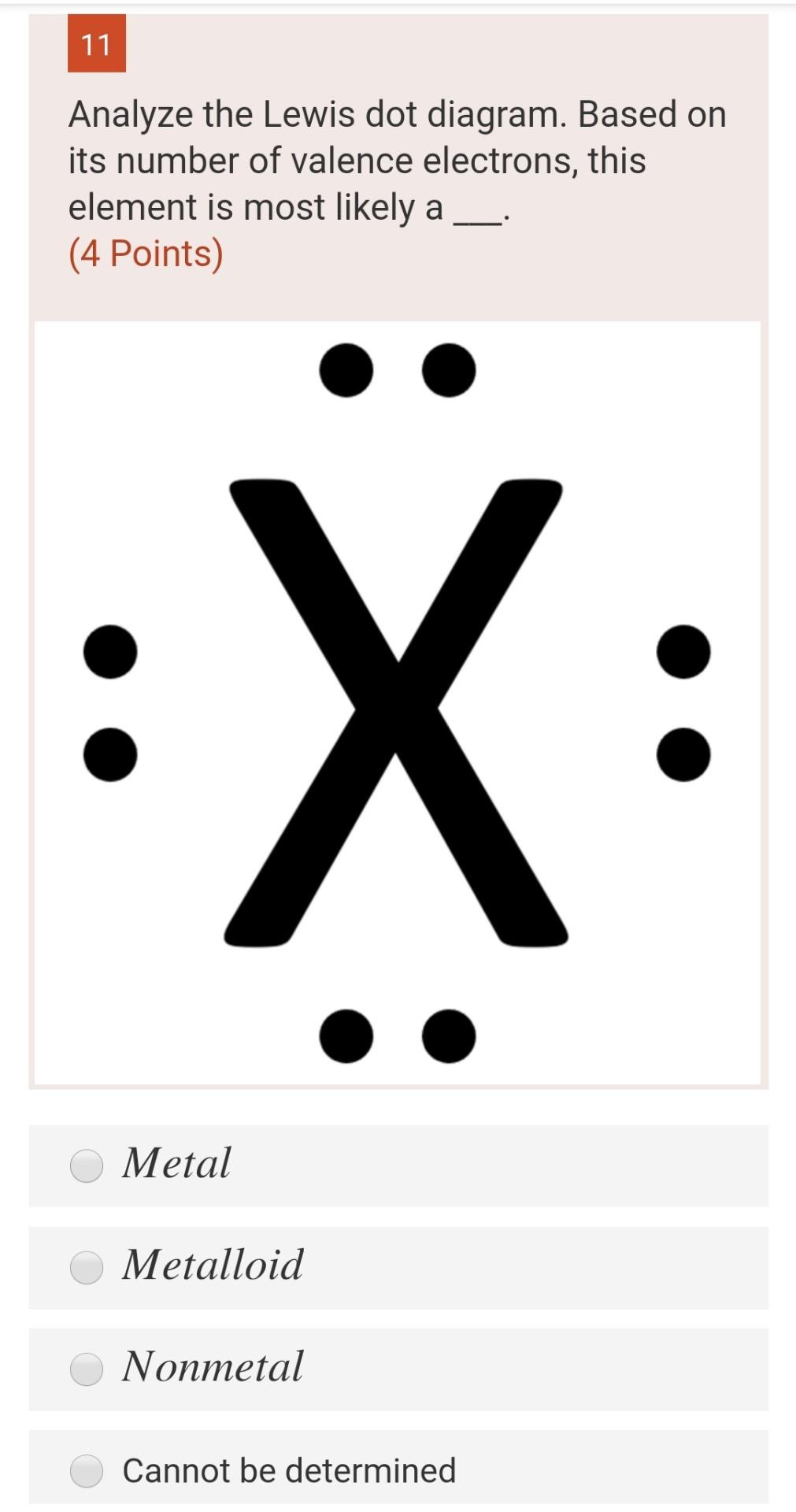
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
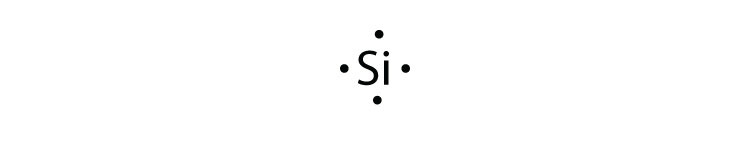
Tin electron dot diagram
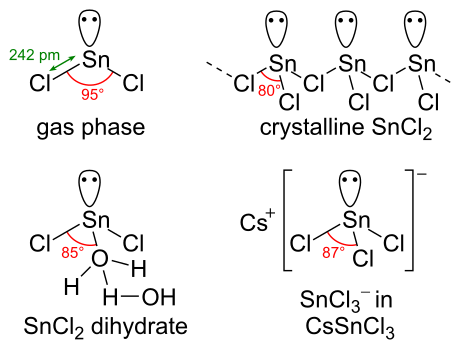
A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence ... Tin (Sn) has an atomic mass of 50. ... chemical and physical properties, states, energy, electrons, oxidation and more. ... Lewis Dot Diagram of Tin (Sn). A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
Tin electron dot diagram. 21 Sep 2018 — Tin Electron Configuration: Tin is a chemical element that has the symbol Sn (It is taken from the Latin word tannum). Oct 22, 1995 · It is also used in the manufacture of super conducting magnets. While tin has many uses in alloys, it has few uses in it's pure elemental form. Additional Notes: Coefficient of linear thermal expansion/K-1 alpha 5.3E-6; beta 21.2E-6. Tin Menu. Tin Page One. Overview of Tin; Tin's Name in Other Languages; Atomic Structure of Tin; Chemical ... Electron Distributions Into Shells for the First Three Periods. A chemical element is identified by the number of protons in its nucleus, and it must ... Academia.edu is a platform for academics to share research papers.
(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol ... Collected in The Entire Predicament (Tin House Books, 2007): >I get into my seat pretty early and look at my book here and there, but mostly feel edgy to see if I'll luck out and keep my elbow room, the plane filling up and my heart beating in its cozy pocket with the suspense as seats fill but not next to me yet. A man hunches in the aisle, leaning his forehead on his fists on the overhead compartment, paunchy and affable. His kids are up there and he's back here. I express my sorrow. Blowe... A step-by-step explanation of how to draw the SnF2 Lewis Dot Structure.For the SnF2 structure use the periodic table to find the total number of valence elec... Jan 08, 2009 · There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for ...
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. Tin (Sn) has an atomic mass of 50. ... chemical and physical properties, states, energy, electrons, oxidation and more. ... Lewis Dot Diagram of Tin (Sn). A covalent bond is a chemical bond that involves the sharing of electron pairs between atoms.These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding. For many molecules, the sharing of electrons allows each atom to attain the equivalent of a full valence ...

Development Of Tin Oxide Materials As A Highly Efficient Electron Transport Layer For Perovskite Solar Cells Semantic Scholar

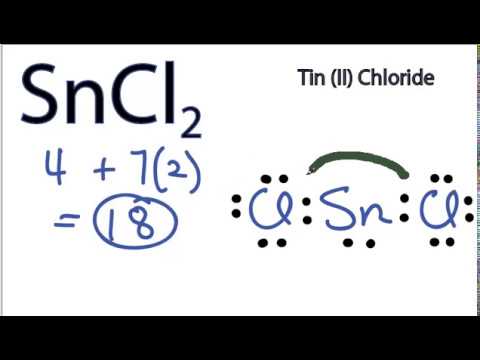





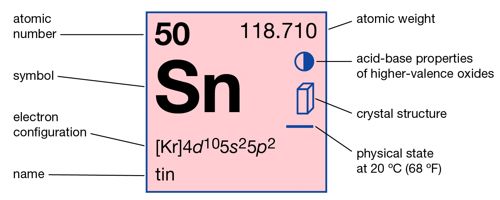

















0 Response to "43 tin electron dot diagram"
Post a Comment