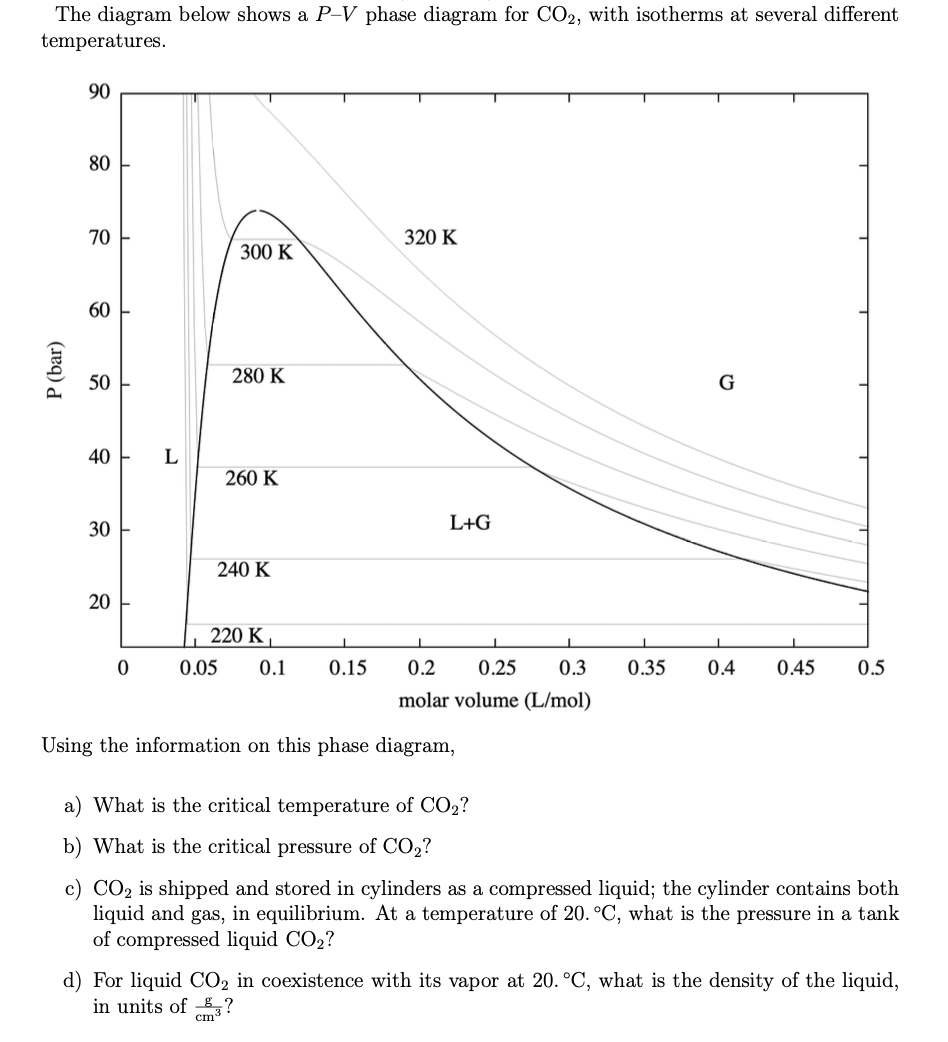
40 phase diagram for co2
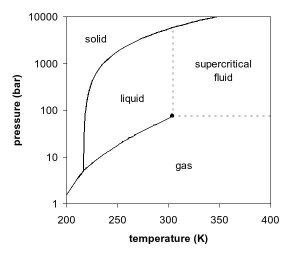
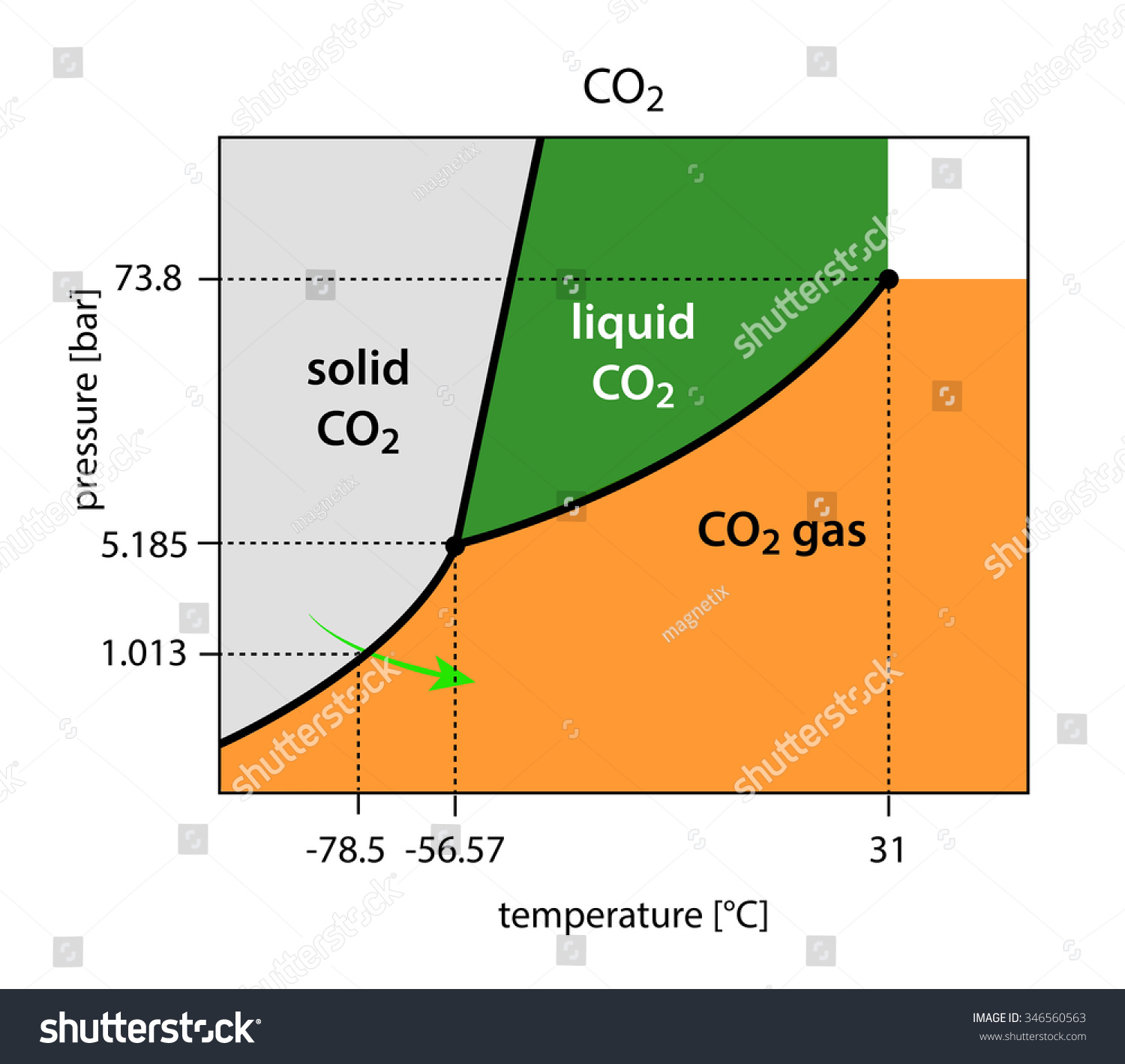
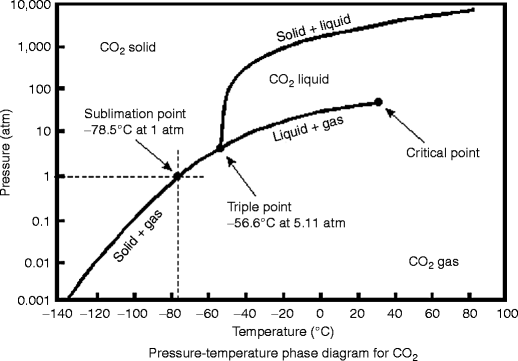
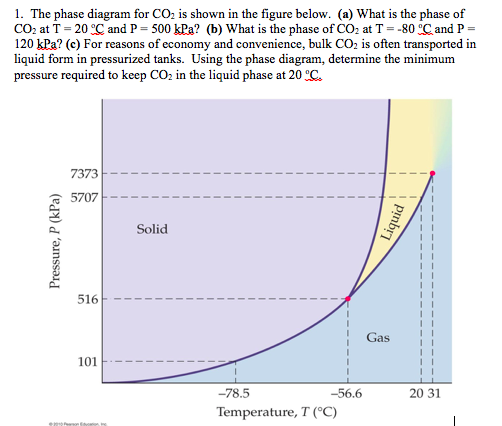
Consider the phase diagram for carbon dioxide shown in Figure 5 as another example. The solid-liquid curve exhibits a positive slope, indicating that the melting point for CO 2 increases with pressure as it does for most substances (water being a notable exception as described previously). Notice that the triple point is well above 1 atm, indicating that carbon dioxide cannot exist as a liquid ...
25.10.2016 · Figure 5-4: (a) Phase Diagram of Ethane-normal Heptane [1], (b) Critical Loci for Binary Mixture. Extracted data from the graphs such as Figure 5‑4 indicate conditions at which mixture of binary pairs are miscible. To have a miscibility of two compounds of ethane and normal pentane with any composition, at each temperature the pressure should be higher than the …
Phase diagram for carbon dioxide 0 10 20 30 40 50 60 70 80 90-80 -60 -40 -20 0 20 Temperature/oC Pressure / atm liquid gas solid bp line mp line subl line “Vapour pressures … above one atmosphere”. In fact, the vapour pressure data start at page 215 for a large number of organic and inorganic substances, and preceding that, for various ...

Phase diagram for co2
This chemistry video tutorial explains the concepts behind the phase diagram of CO2 / Carbon Dioxide and the phase diagram of water / H2O. This video contai...
Carbon dioxide phase diagram. Chemical, physical and thermal properties of carbon dioxide: Values are given for gas phase at 25 o C /77 o F / 298 K and 1 atm., if not other phase, temperature or pressure given. For full table with Imperial Units - rotate the screen!
Carbon Dioxide: Temperature - Pressure Diagram S a t u r at i o n Li ne. Title: phase_diagram.xls Created Date: 11/10/1999 5:44:57 PM
Phase diagram for co2.
by E Goos · 2011 · Cited by 52 — Phase diagrams of carbon dioxide, nitrogen and their mixtures with different amounts of nitrogen (e.g. 5 mol%, 10 mol% N2) were calculated with high ...8 pages
CO2 stands for carbon dioxide. It is some kind of gas in chemical formula name which is composed with two atoms of oxygen double bonded into one atom of carbon. The simple way to say is when you breathe an air which is O2 (oxygen) you breathe out a CO2 (carbon dioxide). The following diagrams are the example of its phase.
R22 P/H Diagram * Diagram created using REFPROP – NIST Reference Fluid Properties As can be seen, the critical point of R-22 is more than 200°F, placing it well above the operating conditions of typical refrigeration systems. This can be contrasted with the CO 2 PH diagram, with a critical point of 88°F. This temperature is often exceeded when ambient air is used as …
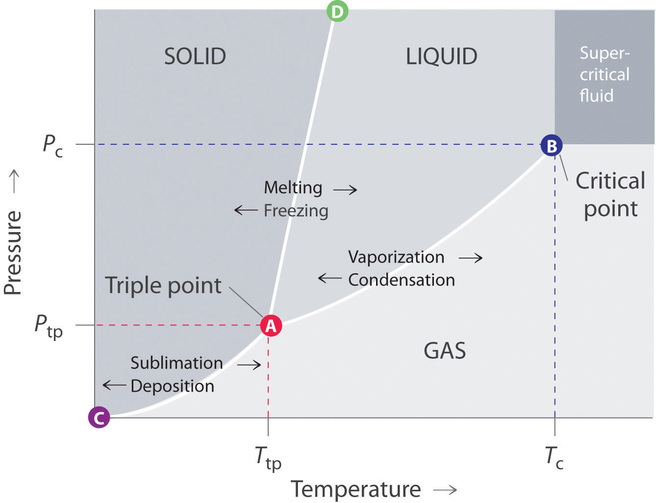
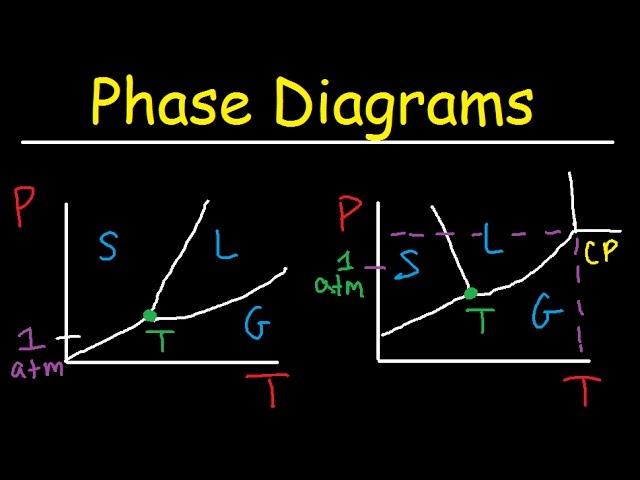
Phase Diagram for a Substance: The figure highlights the critical point, above which (in either temperature or pressure) the substance does not exist in either the liquid or gas phase. Under those conditions it is called a “supercritical fluid,” and has properties between those of a liquid and a gas. It can effuse through solids (like a gas), and dissolve materials (like a liquid). In ...
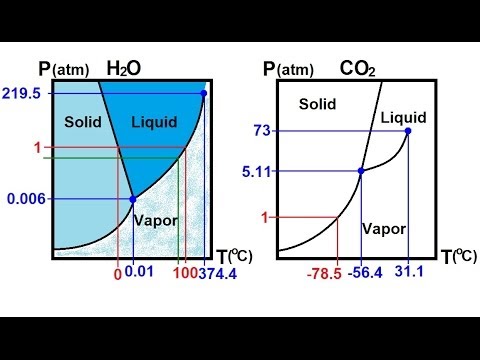
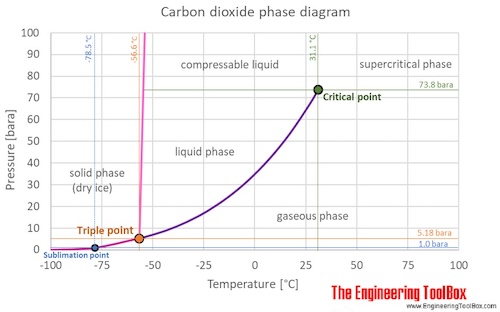
07.06.2018 · The phase diagram of CO2 is similar to the phase diagram of water: sublimation curve, vaporization curve, triple point, critical temperature and pressure.Of course, the P and T values of are unique to carbon dioxide. Solid CO2 is more dense than liquid CO2. Solid CO2 sublimes when heated. Liquid CO2 can be produced only at pressures above 5atm.
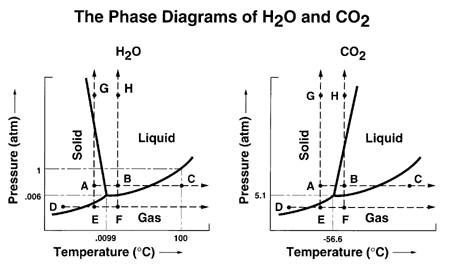
phase diagrams for carbon dioxide and water are shown in figure 1. A phase diagram shows the temperatures and pressures at which the various phases (i.e., solid, liquid and vapor) of a substance can exist. Both phase diagrams for water and carbon dioxide have the same general Y-shape , just shifted relative to one another.
by J Longhi · 2006 · Cited by 60 — The phase diagram shows a lower temperature limit of ∼130 K for clathrate. At this temperature, however, condensation of solid CO2 would have ...
The phase diagram of CO2 is shown below: F1 Puja J 29.4.21 Pallavi D20. The correct statement(s) about CO2 is/are: · At Tc, it can exist in all three phases.1 answer · Top answer: "Concept: A phase diagram is a representation of states of a substance according to varied temperature and pressure. Generally, Temperature is kept ...
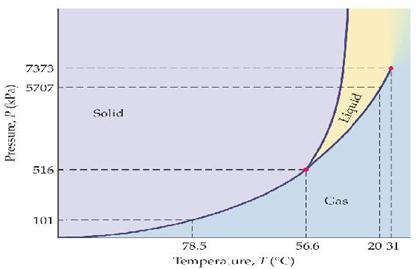
Phase diagram showing the supercritical region (light blue) of carbon dioxide. Fluids suitable for supercritical drying include carbon dioxide (critical point 304.25 K at 7.39 MPa or 31.1 °C at 1072 psi) and freon (≈300 K at 3.5–4 MPa or 25–0 °C at 500–600 psi). Nitrous oxide has similar physical behavior to carbon dioxide, but is a powerful oxidizer in its supercritical state ...
Supercritical carbon dioxide (s CO 2) is a fluid state of carbon dioxide where it is held at or above its critical temperature and critical pressure.. Carbon dioxide usually behaves as a gas in air at standard temperature and pressure (STP), or as a solid called dry ice when cooled and/or pressurised sufficiently. If the temperature and pressure are both increased from STP to be at …
The Phase Diagram of Carbon Dioxide — The Phase Diagram of Carbon Dioxide. In contrast to the phase diagram of water, the phase diagram of CO ...Introduction · The Phase Diagram of Water · The Phase Diagram of Carbon...
19)The phase diagram of a substance is given above. The region that corresponds to the solid phase is _____. A)w B)x C)y D)z E)x and y 19) 20)The normal boiling point of the substance with the phase diagram shown above is _____ °C. A)10 B)20 C)30 D)40 E)50 20) 21)The phase diagram of a substance is shown above. The area labeled _____ indicates ...
16.02.2021 · Using the phase diagram for carbon dioxide below, explain what phase carbon dioxide is normally in at standard temperature and pressure, 1 atm and 273.15 K. Phase diagram for CO2.from Wikipedia. 2: Looking at the same diagram, we see that carbon dioxide does not have a normal melting point or a normal boiling point. Explain what kind of a change carbon …
Use the phase diagram for CO2 in the interactive activity and determine which of the following statements are correct. CO2 is a gas under normal conditions of temperature and pressure. All three phases of CO2 exist simultaneously at the triple point. When the pressure is 4 atm and the temperature is more than −56.7∘C, CO2 exists as a solid.
According to the phase diagram of carbon dioxide, under those conditions of temperature and pressure the solvent lies on the supercritical region [16] . The ...
by E Goos · 2011 · Cited by 52 — Phase diagrams of carbon dioxide, nitrogen and their mixtures with different amounts of nitrogen (e.g. 5 mol%, 10 mol N2) were calculated with high accuracy ...
Phase Diagram for CO2 Phase Diagram for CO2 Phase Diagram for H2O The Liquid State • Vapor pressure no•S teciasfneru •Viscosity •Adhesive/cohesive forces • Capillary action •Density •Compressibility •Diffusion •Evaporation Density of Ice and Water Compressibility Surface Tension Equilibrium Vapor Pressure Vapor Pressure Curves Trouton’s Rule
![Pressure And Temperature Phase Diagram Of Co2 [158,159] - Free ...](https://www.pngkey.com/png/detail/687-6879952_pressure-and-temperature-phase-diagram-of-co2-158.png)




















0 Response to "40 phase diagram for co2"
Post a Comment