41 molecular orbital diagram of h2
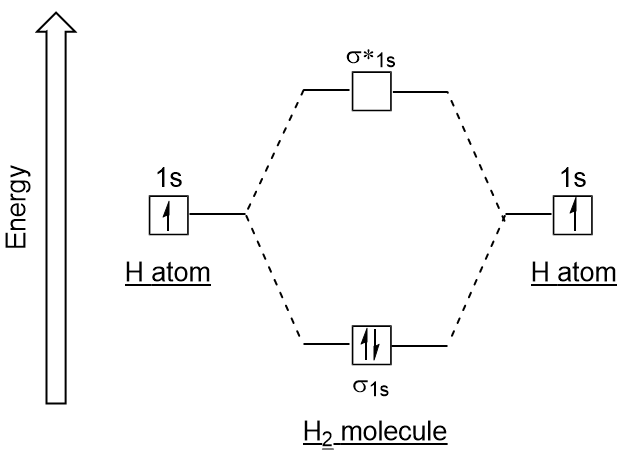
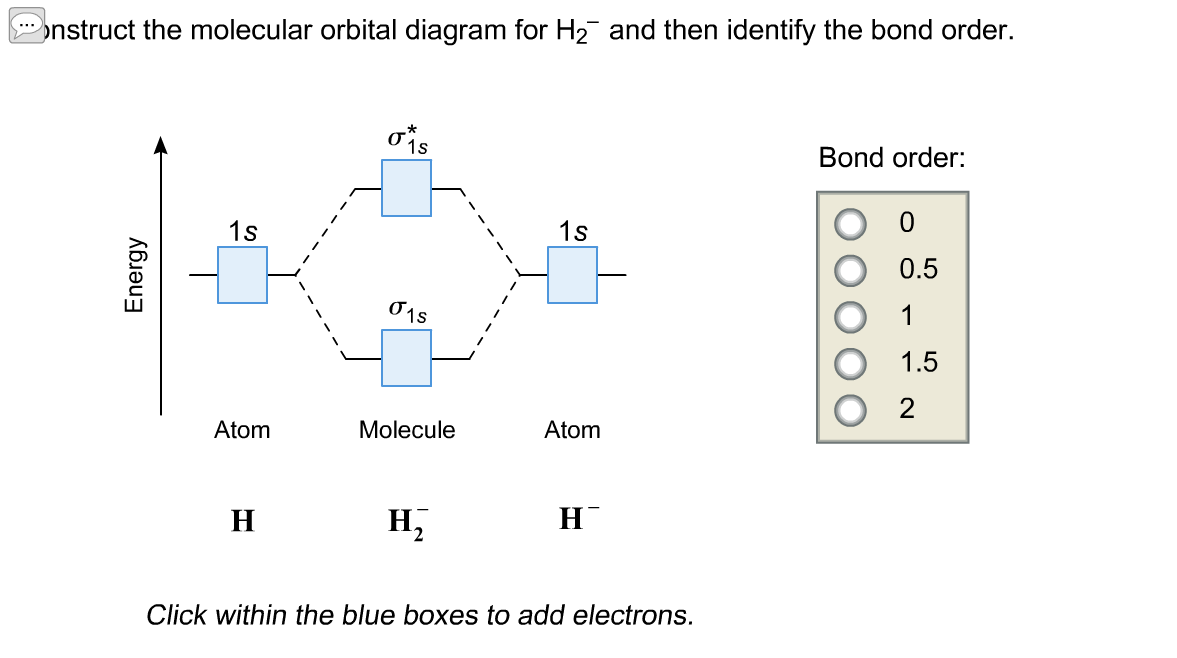
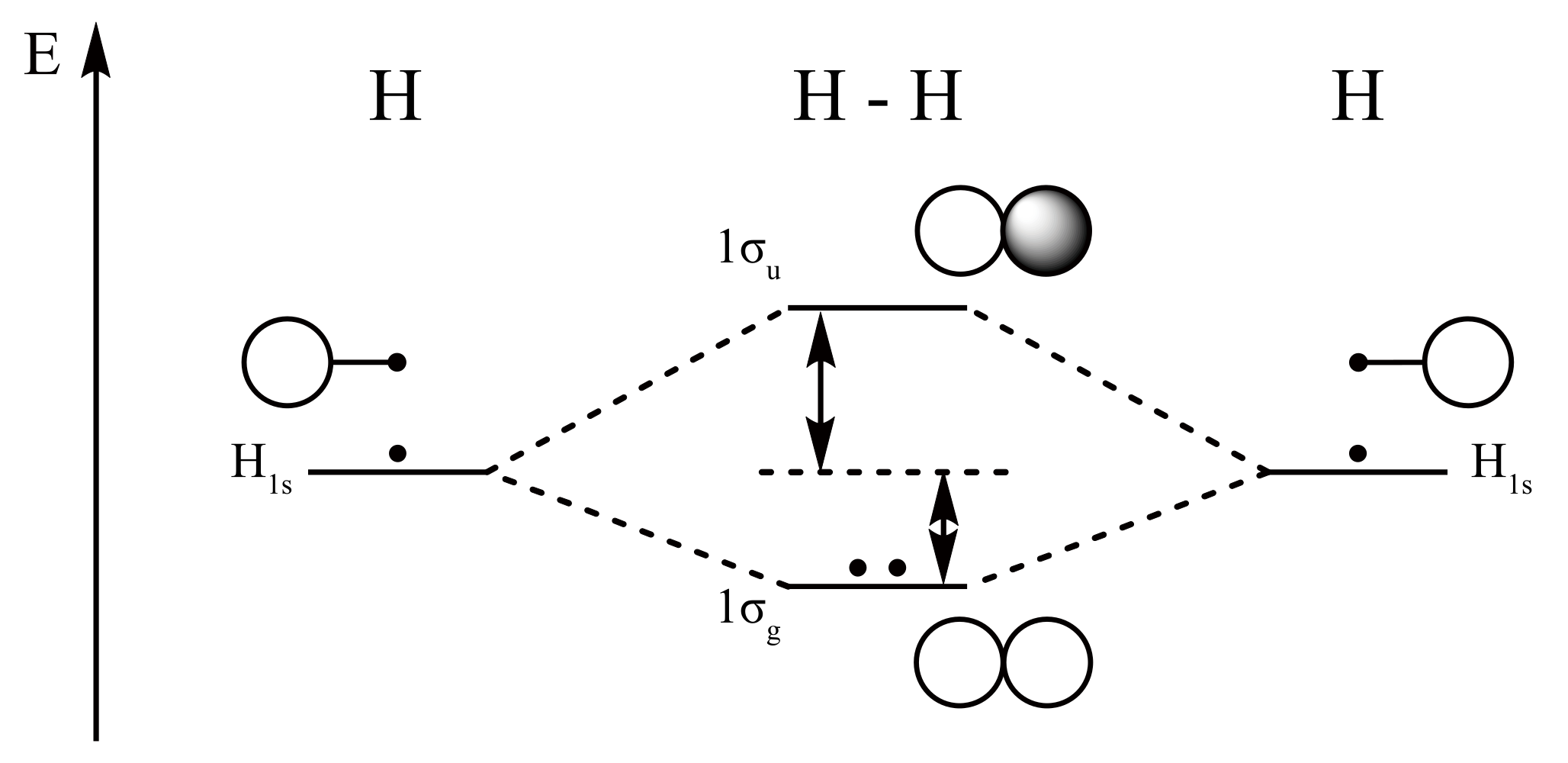
Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, H− 2 has three electrons while H+ 2 has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one σ1s and one σ* 1s MO by conservation of orbitals.
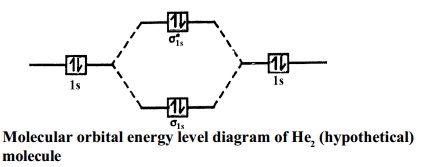
diagram suggests that the energy of an H2molecule is As a result, the H2molecule is more stable than a pair of isolated atoms. Using the Molecular Orbital Model to Explain Why Some Molecules Do Not Exist This molecular orbital model can be used to explain why He2molecules don't exist. electrons in both the bonding and the *
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem
Molecular orbital diagram of h2
In He2 molecule, Atomic orbitals available for making Molecular Orbitals are 1s from each Helium. And total number of electrons available are 4. Molecular Orbitals thus formed are:€1s2€*1s2 It means 2 electrons are in bonding molecular orbitals and 2 are in antibonding molecular orbitals .
14+ H2 Molecular Orbital Diagram. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals.
A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
Molecular orbital diagram of h2.
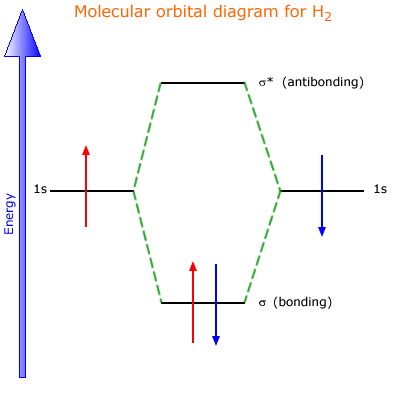
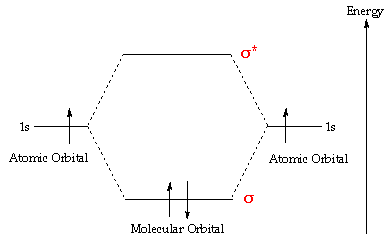
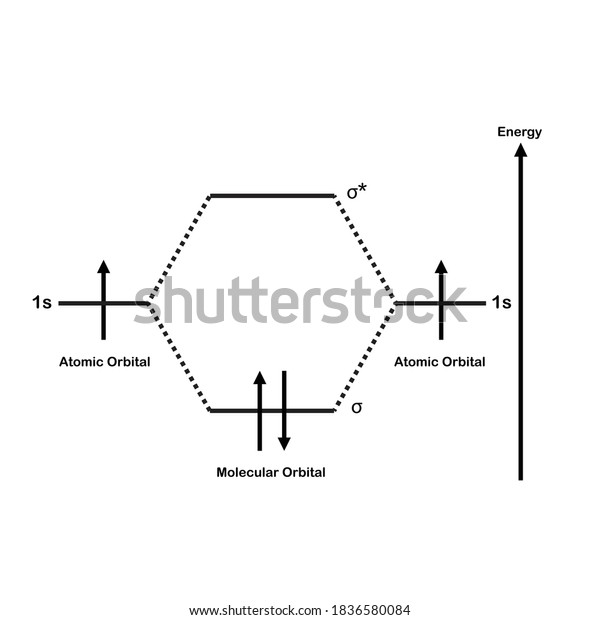
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
Molecular orbital diagram of h2 Answer General guidance Concepts and reason The bonding and anti - bonding interaction of the molecules can be explained with the help of a molecular orbital diagram. It also gives a detailed description of bonding in molecules. Bond order represents the number of bonds present in between two bonded atoms.
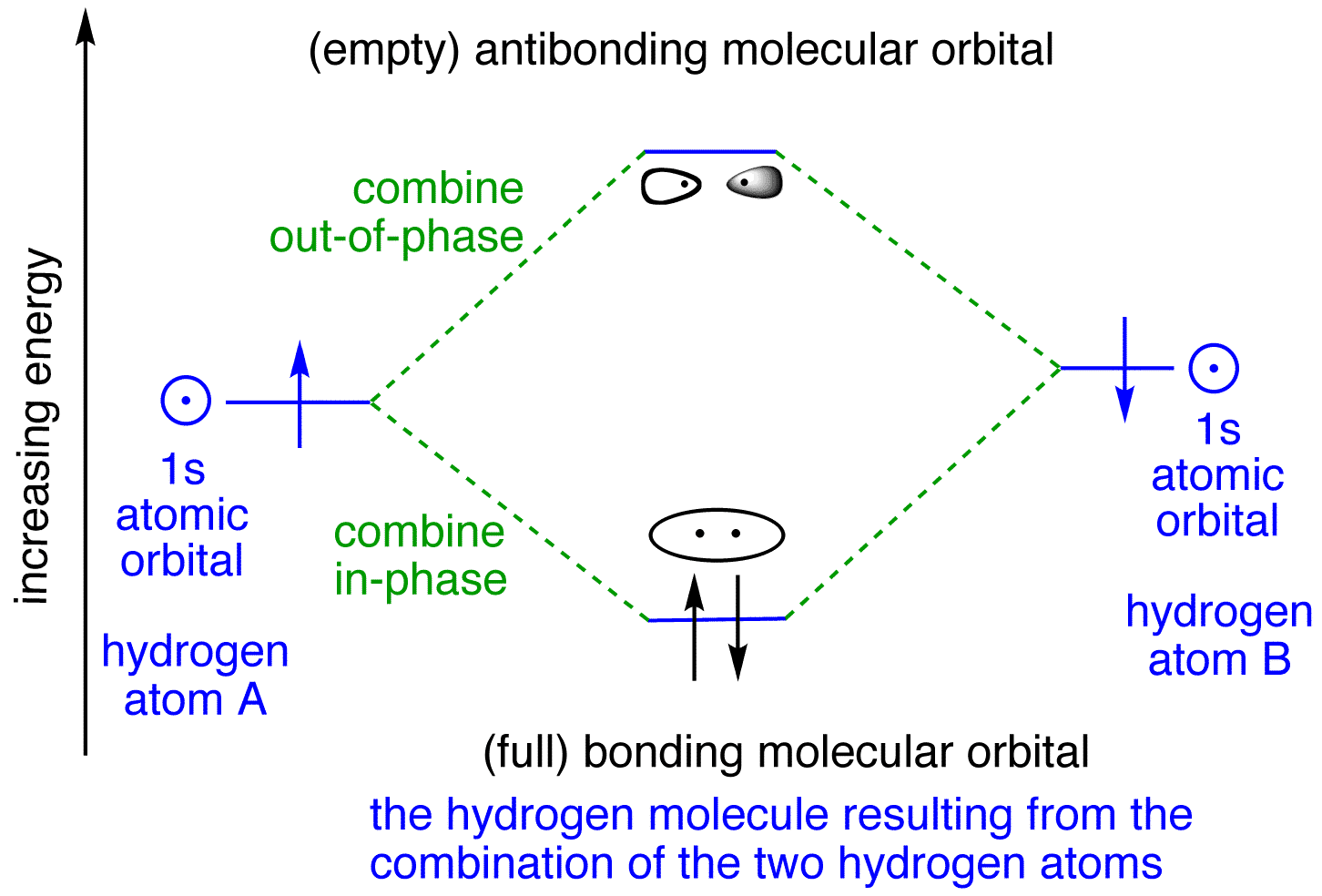
Hydrogen Molecular Orbital Diagram Atomic hydrogen has 1 electron in a 1s orbital. Of course, there are 2s, 2p, 3s, 3p, etc. empty orbitals at higher energy. Let's just consider the 1s orbitals. Remember that an orbital is a mathematical function that describes the probability of finding an electron in space.
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Molecular orbitals of H. The simplest neutral molecule is molecular hydrogen, H 2, which consists of two electrons and two protons. The molecular orbital Hamiltonian in this case is the same as it is for the molecular hydrogen ion and the molecular orbitals are the same as for the molecular ion. HH2 mo =− ℏ2 2me ∇2− e2 4πϵ0|.
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
of the orbitals is specified. b. Molecular Orbital Picture We are now in a position to discuss the basic principles of the molecular orbital (MO) method, which is the foundation of the electronic structure theory of real molecules. The first step in any MO approach requires one to define an effective one electron Hamiltonian, hˆ eff. To this ...
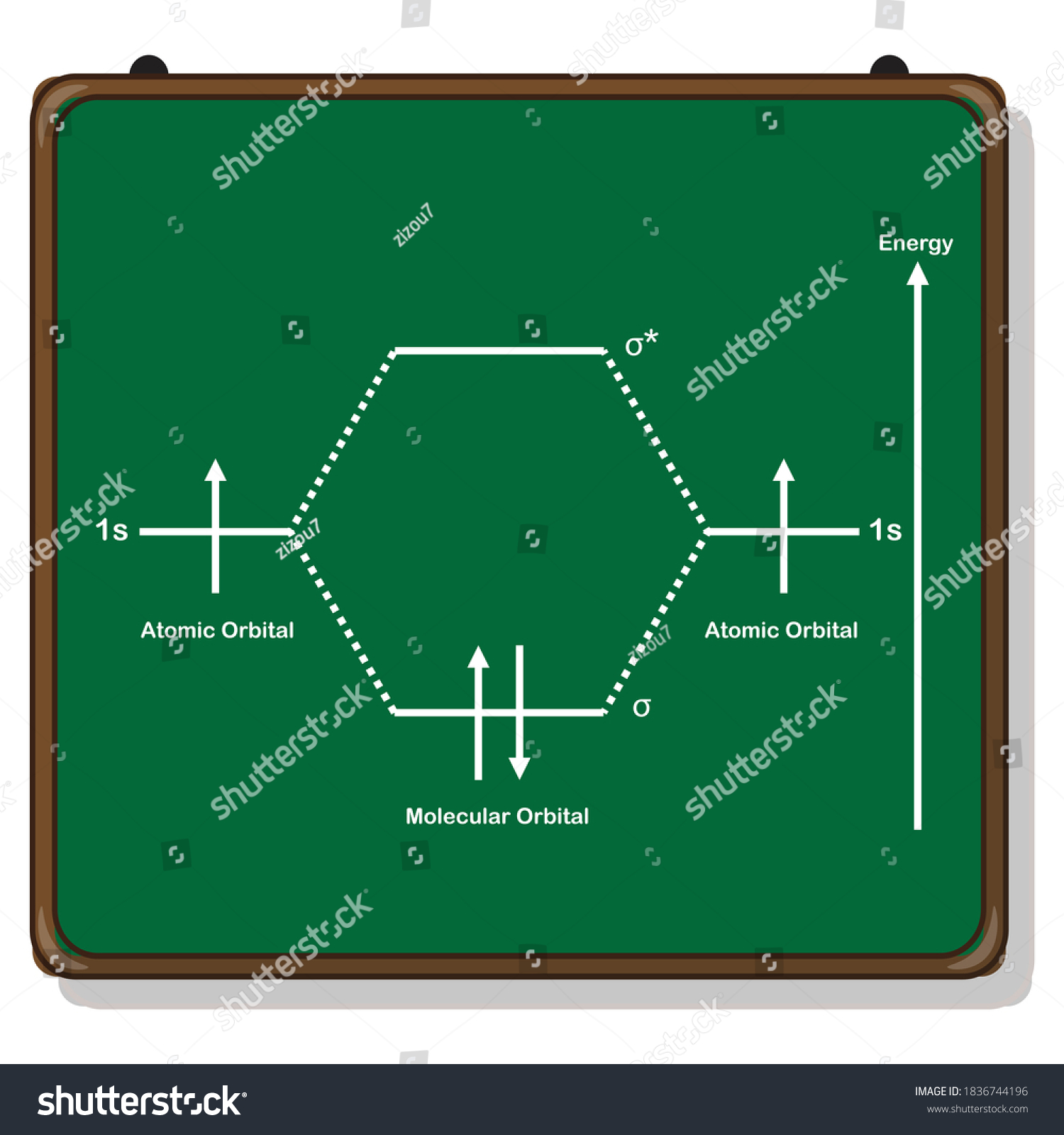
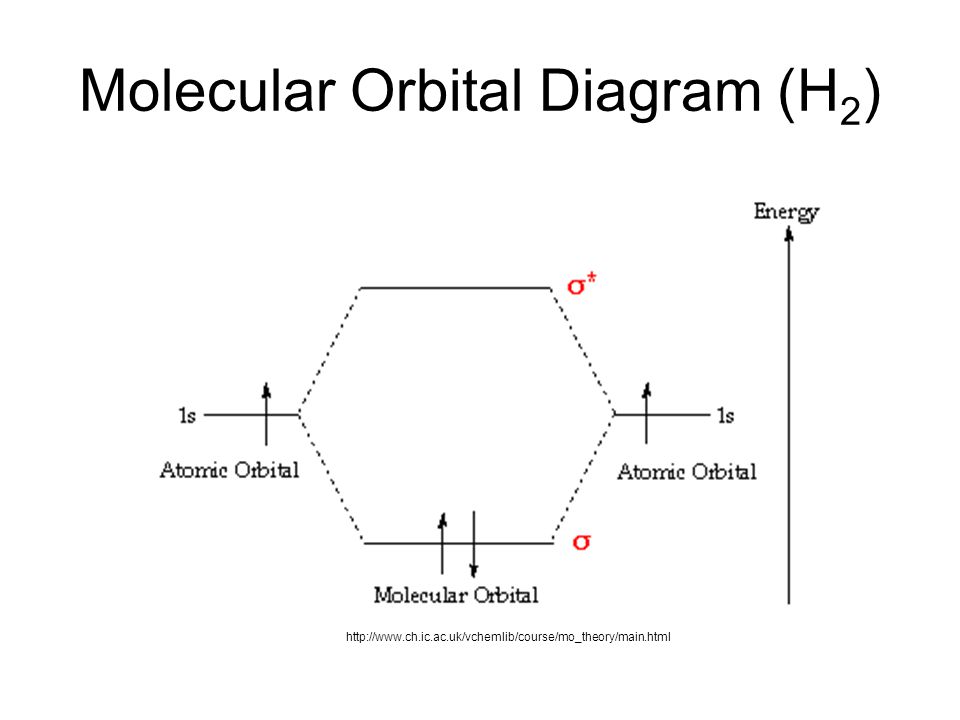
The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H 2 molecule is shown in Figure 13.
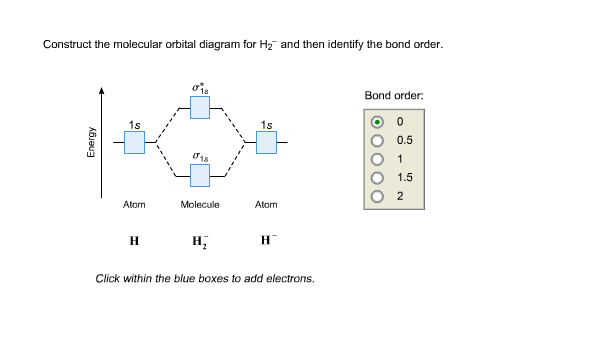
Mo · Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order. chemical bonding molecular orbital s of h2 and he2 as before the greater the number of these nodal planes the more the electrons that occupy the orbital s are excluded from the region between the nuclei and hence the higher the energy the resulting molecular...The energy curves for ψ + and ψ-reveal the ...
Answer to Create an MO diagram for H2 + H2 and H Post the Lumo, lumo -, homo, homo + near its energy level. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2 +, assume the (normalized) ground electronic state wavefunction is . Qualitative MO theory orbital diagram for homonuclear diatomics composed of 1st or.
H2molecule: two 1s atomic orbitals combine to make one bonding and one antibonding molecular orbital. HaHb MOs for H2 • in phase combination • constructive interference • large e-density in the internuclear region (bonding) • an electron in this MO lowers the molecule's energy
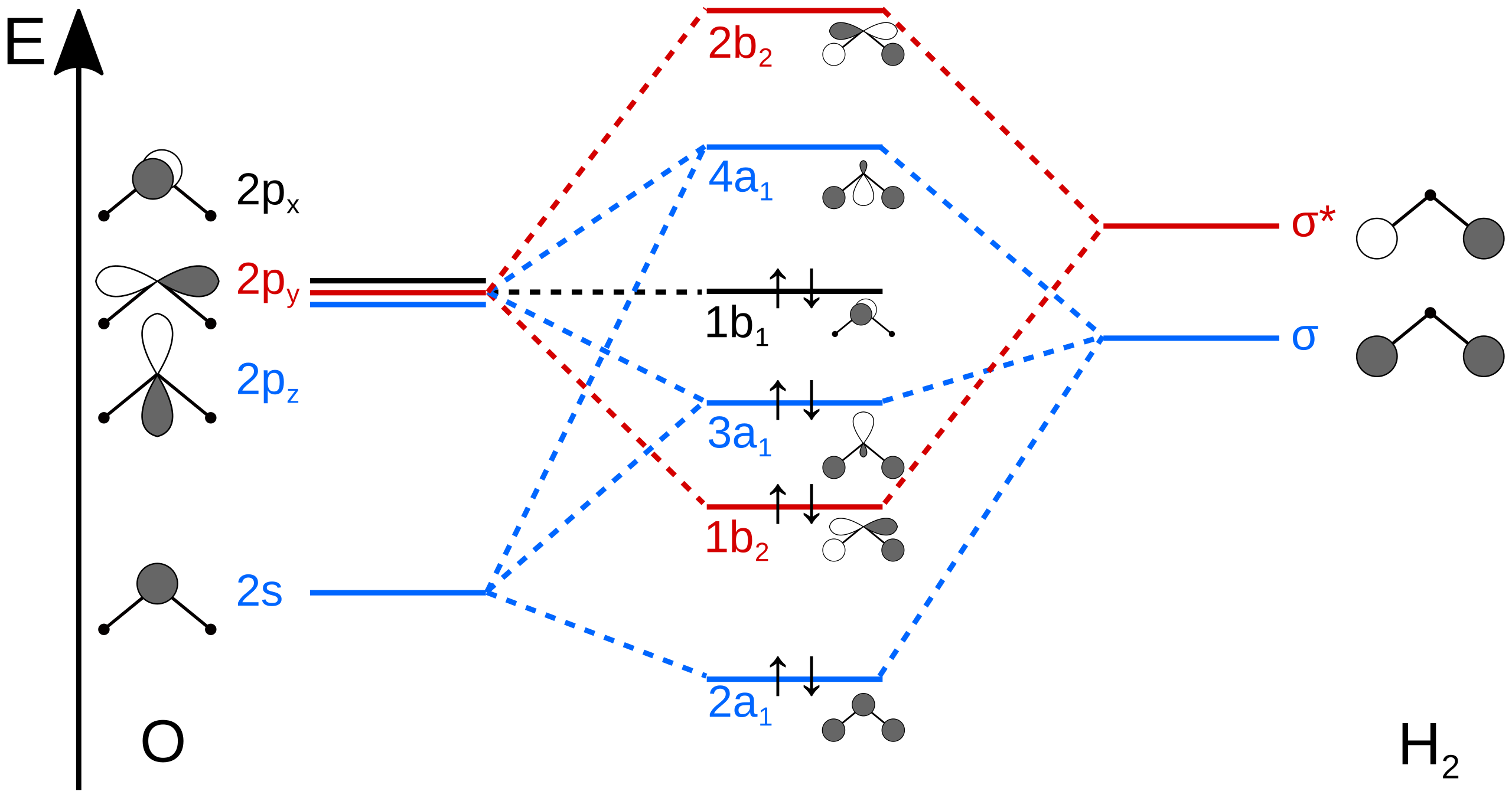
Molecular Orbitals for Water (H 2 O) The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2(2a 1) 2(1b 2) 2(3a 1) 2(1b 1) 2 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set (experimental data is given in [1289]). They are set out with the lowest
Molecular orbital diagram of H2 + . tvewdtamcjFace Reveal 100%Good Meeting!!All are Invited!! 2. ऐलिफैटिक यौगिक क्या है? A positively charged ball hangs from a silk thread. We put a positive test charge q° at a point and measure F/q°, then it can be predicted that the el …. ectric field strength E (a ...
In this video, we take a detailed look at the molecular orbitals of the H2 molecule, with an introduction to molecular orbital diagrams. Discussed in this v...
chemical bonding molecular orbitals of h2 and he2 as before the greater the number of these nodal planes the more the electrons that occupy the orbitals are excluded from the region between the nuclei and hence the higher the energy the resulting molecular orbital energy level diagram is shown alongside the orbitals in the illustration
The Hydrogen Molecule Ion H 2 +. The LCAO method adopts an especially simple form for homonuclear diatomic molecules, i.e. molecules that consist of two identical atoms, e.g. H 2, O 2, N 2.It is recommendable to begin with the most simple among those systems, the hydrogen molecule ion H 2 +.As this molecule has only one electron, this molecule is for a consideration of the chemical bond as ...
Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ...
Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure.
Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic ... Spin‐orbitals of type 1 and 3 have the same symmetry, and therefore can "mix" (to give improved wavefunctions and energy eigenvalues): 1 ψψ αβ ...
Construct the molecular orbital diagram for h2 and then identify the bond order. Each hydrogen atom contributes one electron and thus h2 has three electrons while h2 has one. Discussed in this video are. Draw mo energy diagrams for the molecular ions h2 and h2. Construct the molecular orbital diagram for h2.
H2 Molecular Orbital Diagram MO diagram of dihydrogen Bond breaking in MO diagram The smallest molecule, hydrogen gas exists as dihydrogen (H-H) with a single covalent bond between two hydrogen atoms. As each hydrogen atom has a single 1s atomic orbital for its electron, the bond forms by overlap of these two atomic orbitals.
Dec 15, 2018 · Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here.
Molecular orbital diagram h2. Because of their simplicity they have been extensively studied. Two superpositions of these two orbitals can be formed one by summing the orbitals and the other by taking their difference. Construct the molecular orbital diagram for h2 and then identify the bond order.




















0 Response to "41 molecular orbital diagram of h2"
Post a Comment