44 use the energy diagram for the reaction to answer the questions.
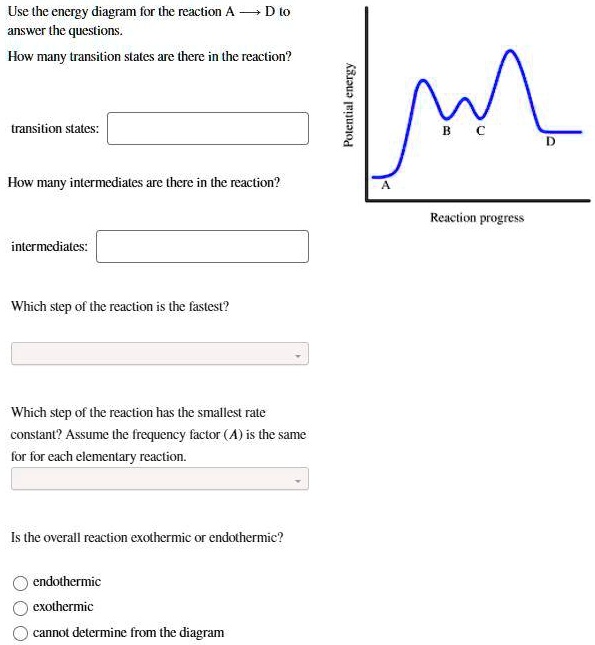
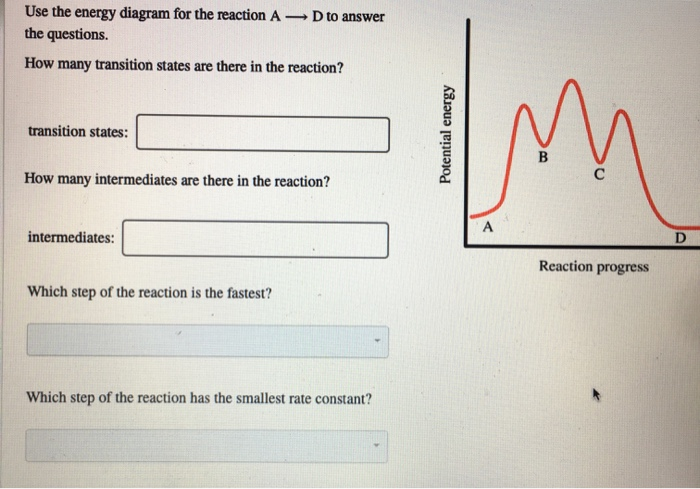
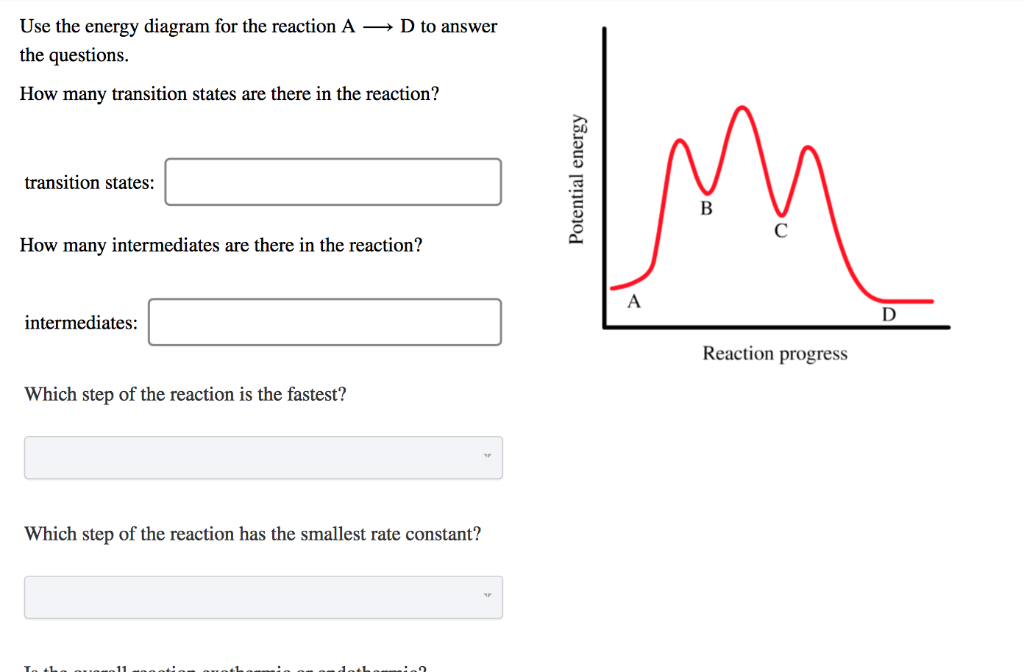
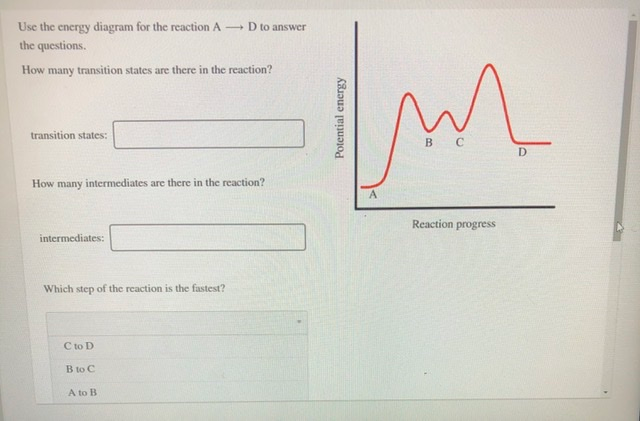
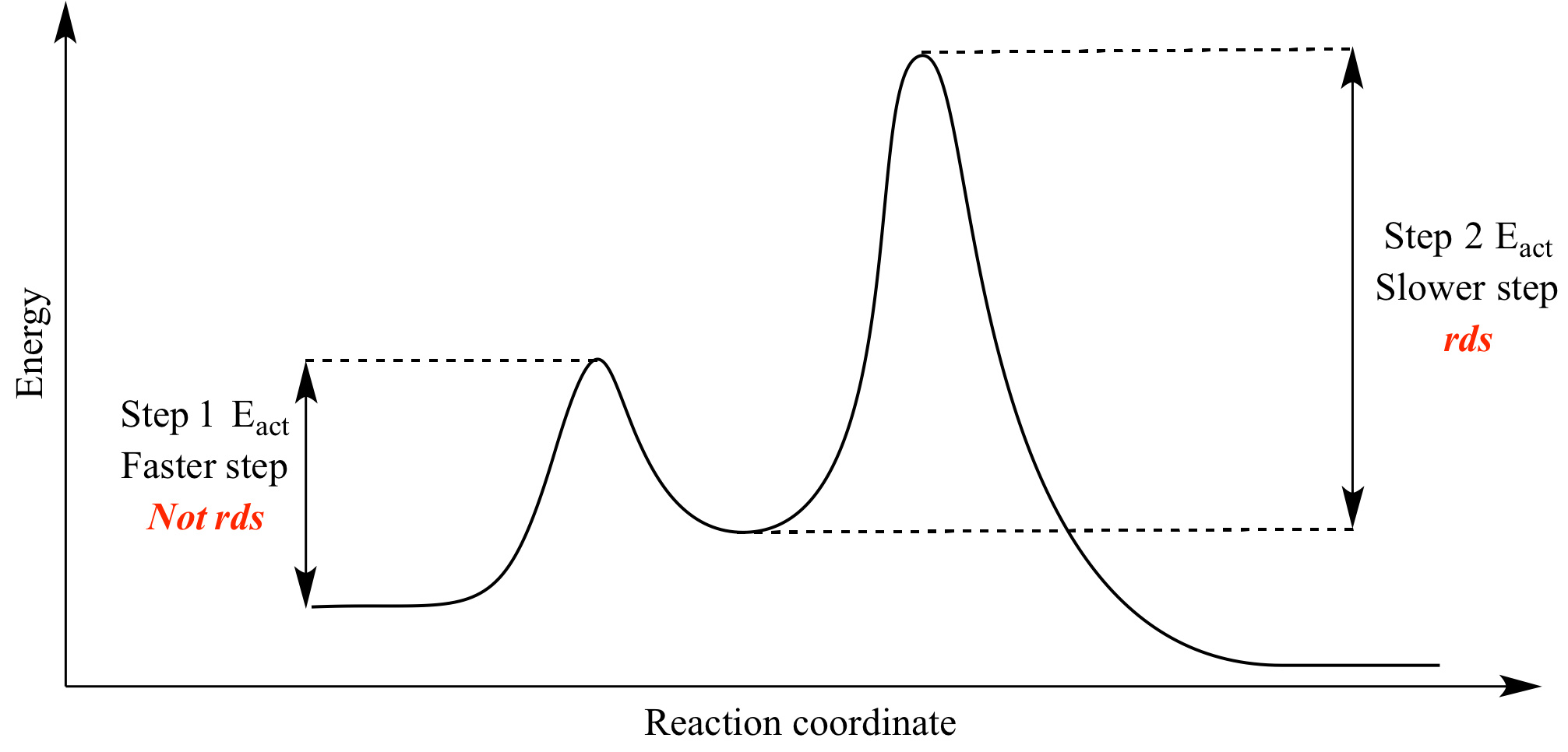
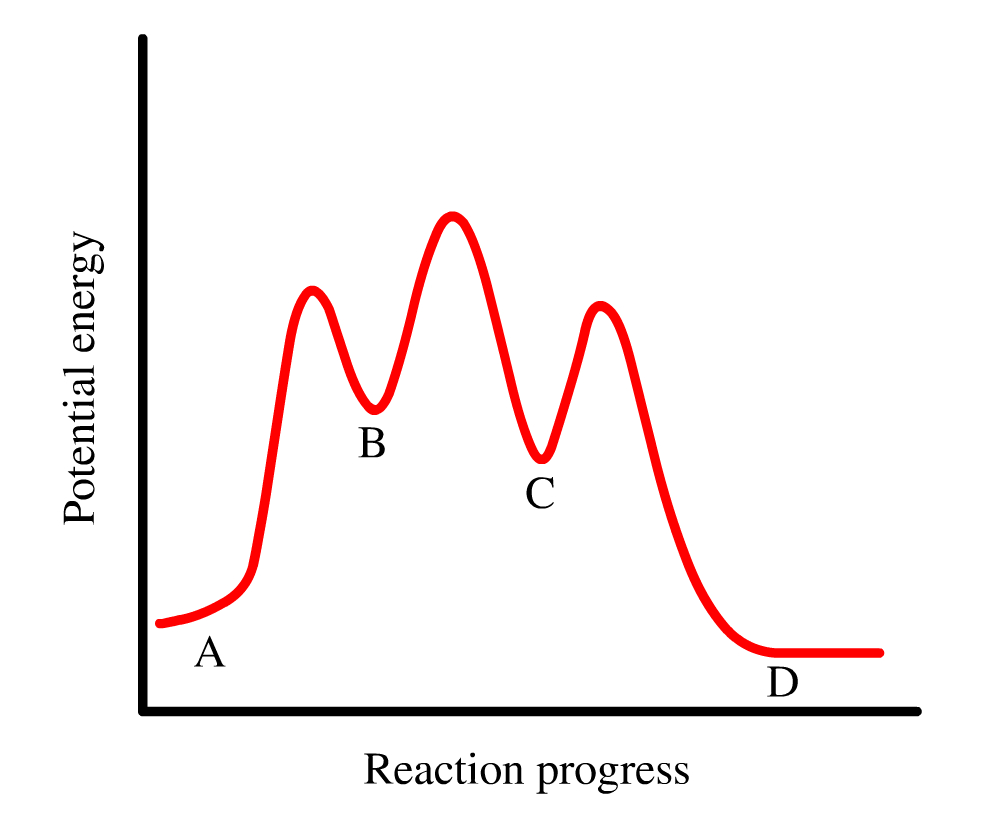
Potential energy. Use the energy diagram for the reaction A → D to answer the questions. How many transition states are there in the reaction? transition states: B How many intermediates are there in the reaction? A D Reaction progress intermediates: Which step of the reaction is the fastest? Potential energy.
Transcribed image text: Use the energy diagram for the reaction AD to answer the questions. How many transition states are there in the reaction?
Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? transition states: Potential energy How many intermediates are there in the reaction? intermediates: Reaction progress Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant?

Use the energy diagram for the reaction to answer the questions.
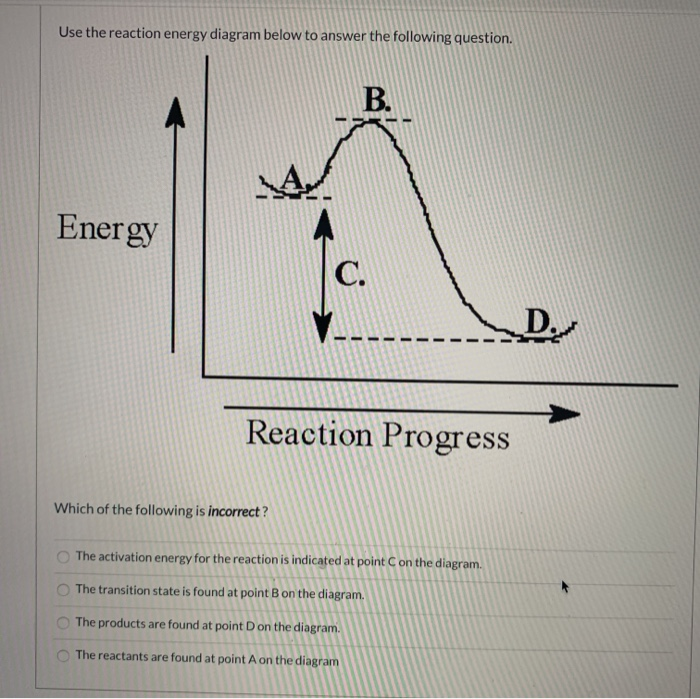
July 14, 2020 - How much energy is required for this reaction to occur? ... B) Reactants are located on the flat portion to the left of the peak. Products are located on the flat portion to the right of the peak. The activated complex is located at the peak of the reaction diagram.
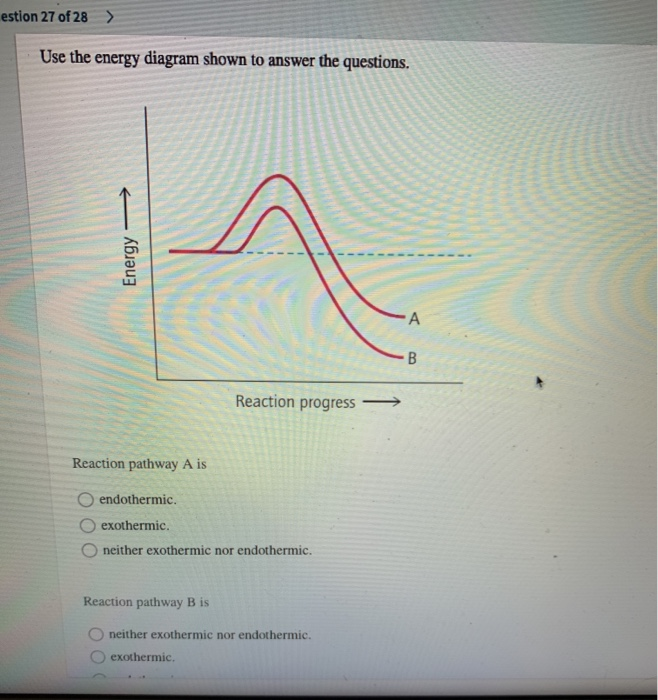
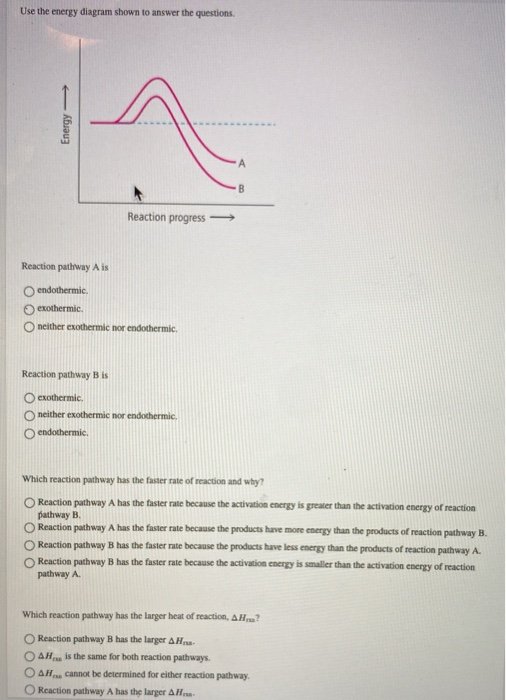
Energy Reaction progress Reaction pathway Ais endothermic exothermic neither exothermic nor endothermic. Reaction pathway B is exothermic. neither exothermic ...
Get ready for your exams with this BBC Bitesize GCSE Chemistry Energy changes (AQA) exam preparation guide.
Use the energy diagram for the reaction to answer the questions..
Use the energy diagram for the reaction A D to answer the questions. How many transition states are there in the reaction? How many intermediates are there in the reaction? Which step of the reaction is the fastest? Which step of the reaction has the smallest rate constant? Assume the frequency factor (?) is the same for each elementary reaction.
Learn how energy changes can be calculated and displayed using energy diagrams with BBC Bitesize GCSE Chemistry.
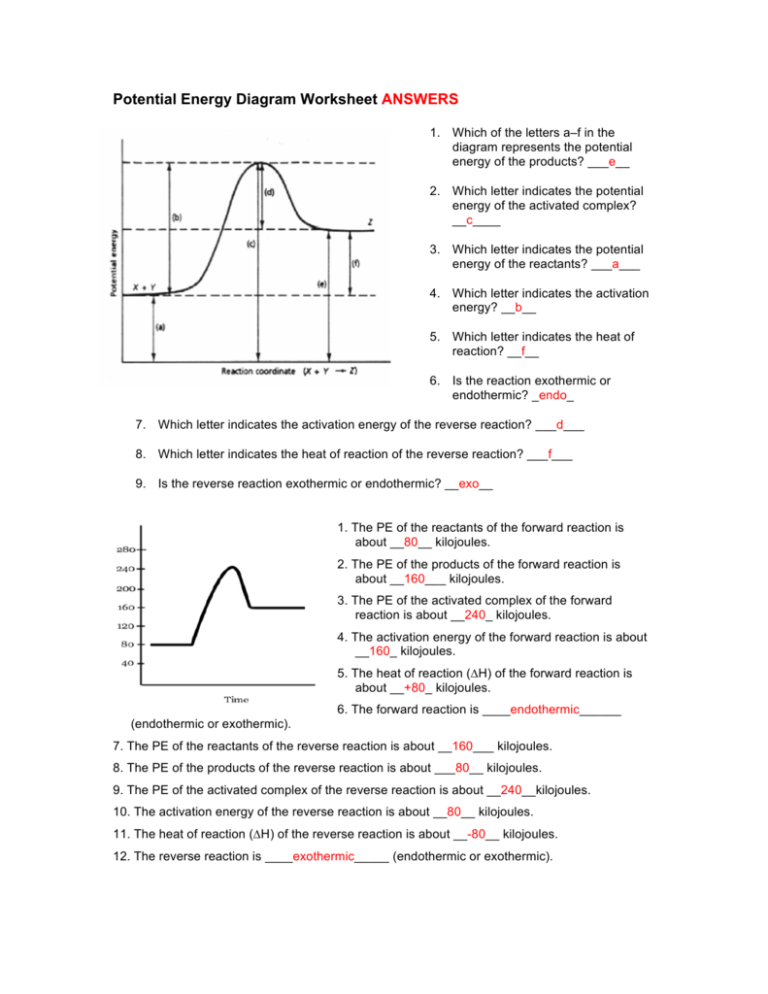
View peds mmm.doc from AA 1Potential Energy Diagrams USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. Is the overall reaction as shown exothermic or endothermic? Endothermic 2. What
Name _____ Potential Energy Diagrams Use the following potential energy diagram to answer the questions below. 1. Is the overall reaction as shown exothermic or endothermic?_____ 2. What is the activation energyfor the forward reaction?_____ 3.
Ask any question and get an answer from our subject experts in as little as 2 hours.
Ask any question and get an answer from our subject experts in as little as 2 hours.
Problem Details. Use the energy diagram for the reaction A → D to answer the questions. Q. Which of the following has ΔG°f = 0 at 25°C?a. O3 (g)b. O (g)c. H2O (g)d. H2O (l)e. Na (s) Q. Predict the sign of ΔG for an endothermic reaction with adecrease in entropy. a)cannot predict b)positive c)negative d)no change e)Aquarius.
Transcribed image text: macmilan learning Use the energy diagram for the reaction A → D to answer the questions. How many transition states are there in ...
Answer with step by step detailed solutions to question from Arihant's JEE Main 2018, JEE Questions- "An element undergoes a reaction as follows, X+2e^-to X^2- and energy released =30.86 eV/atom. If the energy released is used to dissociate 4 g of H2 molecule and equally into H^+ and H ^*, ...
Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? FREE Expert Solution. Recall that an energy diagram is usually read from left to right. The components of a one-step energy diagram are:
Ask any question and get an answer from our subject experts in as little as 2 hours.
Rates, Temperature and Potential Energy Diagrams Worksheet Part 1: 1. Use the potential energy diagram shown to the right to answer the following: a. Label the axis. yaxis is potential energy (kJ or kJ/mol) xaxis is reaction progress b. What does each curve represent? Each curve represents a
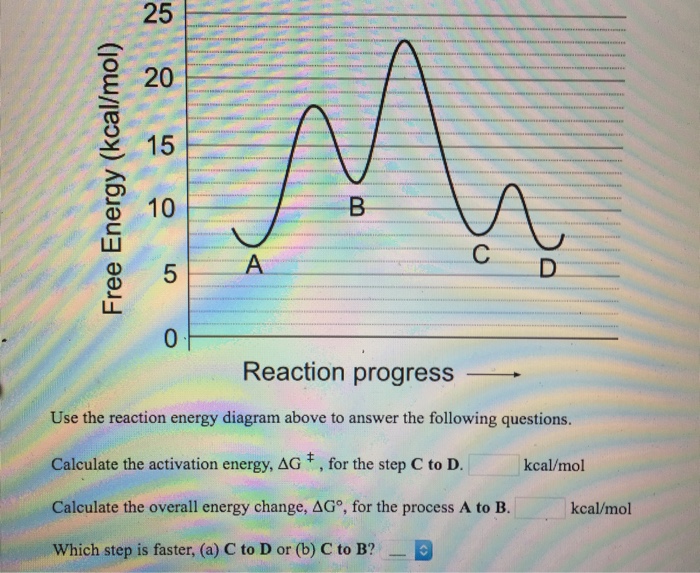
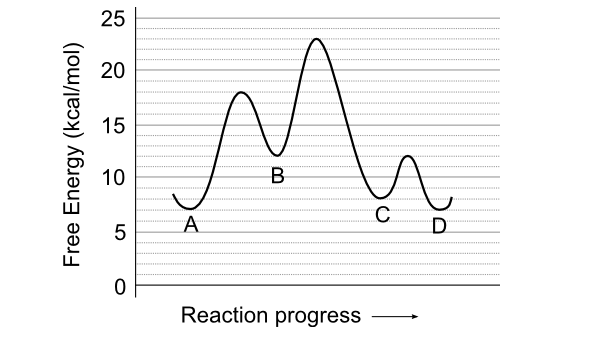
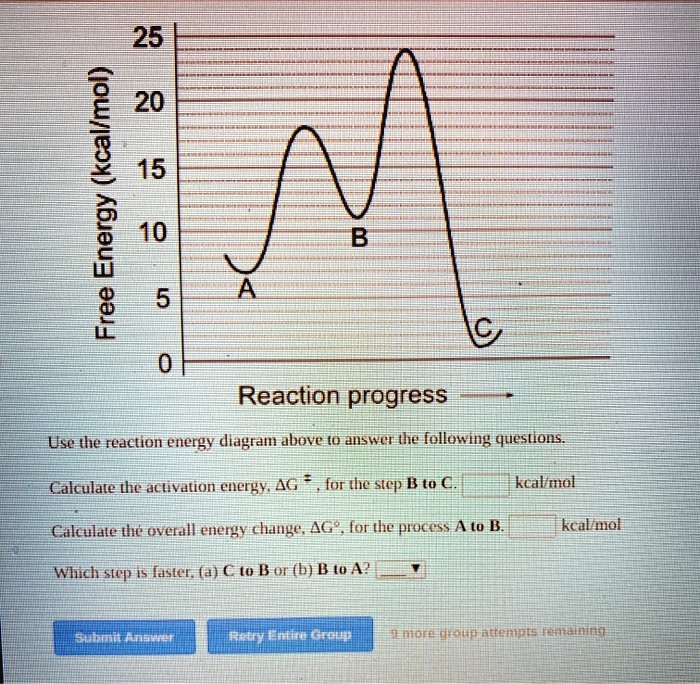
Use the reaction energy diagram above to answer the following questions. For the reverse reaction on the graph above. Calculate the activation energy g for the step c to b kcalmol calculate the overall energy change g for the process b to d kcalmol which step is faster a b to a or b a to b. The arrow labeled c represents a transfer of chemical ...
Potential Energy Diagrams Worksheet CK-12 Foundation Chemistry Name Use the following Potential Energy Diagram to answer questions 1 - 12. 150 X2Y2 100 Potential Energy (kJ) 50 0 Progress of Reaction 1. Is the overall reaction as shown exothermic or endothermic? 2. What is the activation energy for the forward reaction? 3. What is the ...
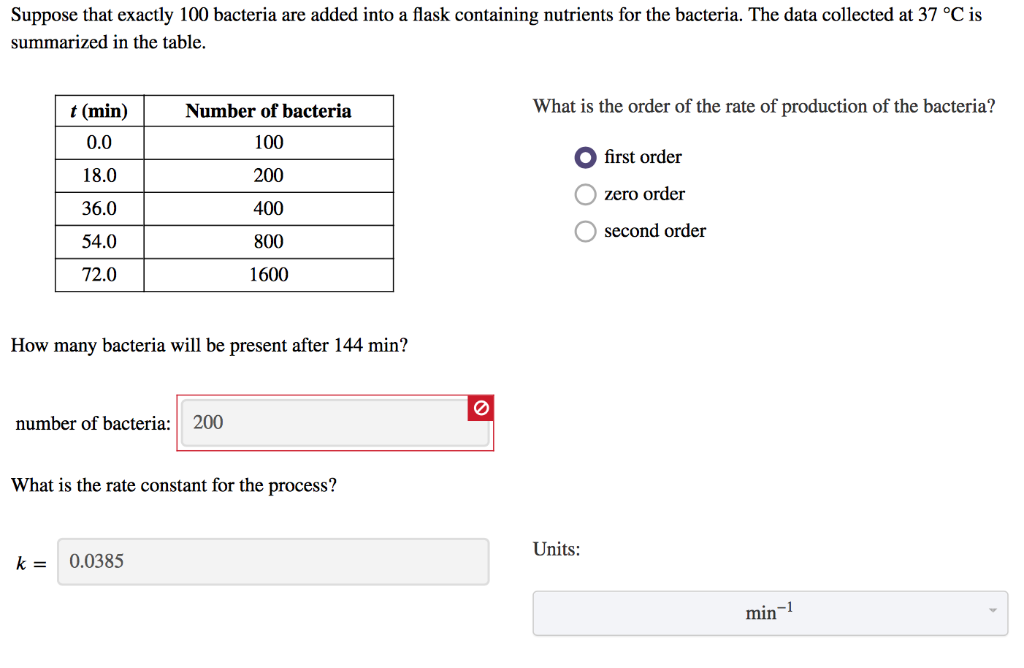
Kv = K° ×e− Ea RT. where K° depends by many factors as solvent, geiometry, type of reaction. Ea is the activation energy. R is the universal costant of gases. T is the absolute temperature. Hence highter is the activation energy, lower is the rate of the reaction. Normally if the activation energy is more than about 10Kcal/mol the reaction ...
Use the potential energy diagram below to answer the following question The potential energy of the reactants is ____kJ, while the potential energy of the products is _____. ( straight, wayyyyyyyyyyy uphill, then tiny downhill, then straight )
Ask any question and get an answer from our subject experts in as little as 2 hours.
The figure above represents the reaction profile of a two step, exothermic reaction. The y-axis represents the potential energy of the reaction species, and the x-axis represents the progress of the reaction. The reaction is exothermic because the energies of the products are lower than those of the reactants. The reactants are represented by the horizontal line at the far left of the graph ...
Look at the two exothermic reactions whose potential energy diagrams are represented in figures A and B below, and notice the activation energy marked in each. When a reaction has a low activation energy, like in figure A above, most of the reactant molecules have sufficient kinetic energy to react, and the reaction will most likely be rapid (a ...
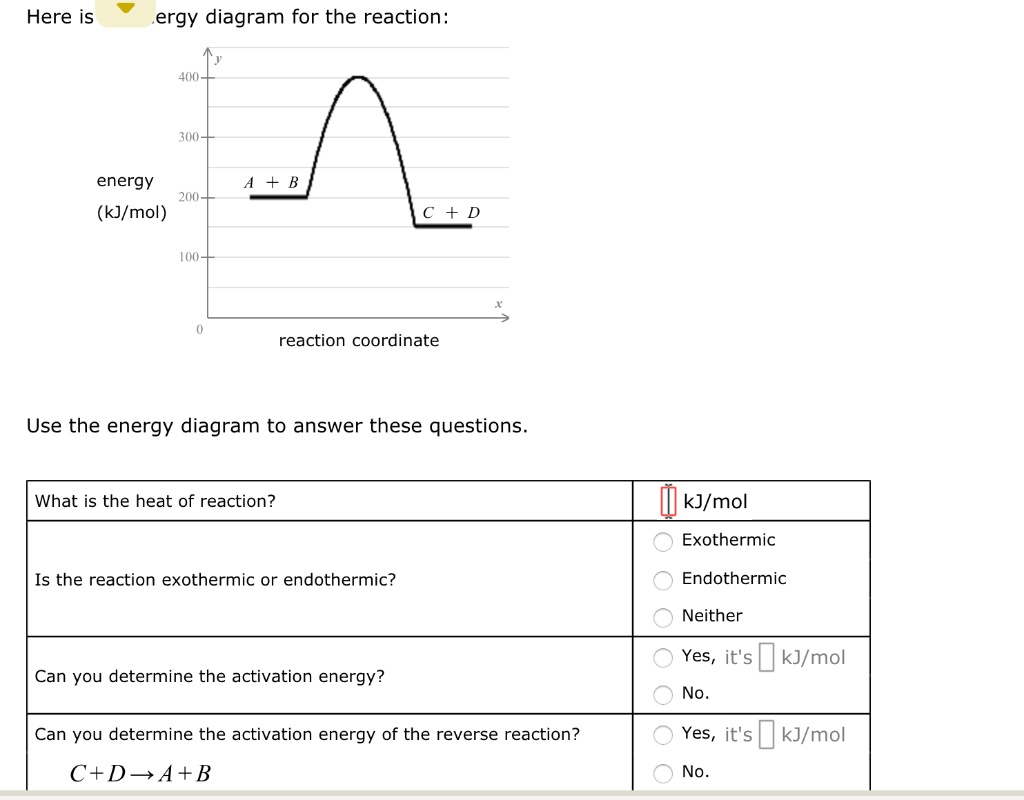
What is the heat of reaction? kJ/mol O Exothermic O Endothermic Neither O Yes, it'skJ/mol Is the reaction exothermic or endothermic? Can you determine the ...
March 16, 2017 - Good Day Forest Hills... Fall 2021 · It is the policy of the New York City Department of Education to provide equal educational opportunities without regard to actual or perceived race, color, religion, creed, ethnicity, national origin, alienage, citizenship status, disability, weight, gender ...
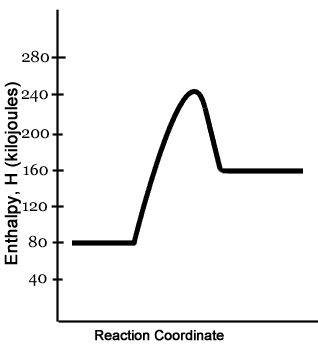
In this reaction, the total energy of the reactants is 80 kJ mol-1, the total energy of the products is -90 kJmol-1 and the activation energy for the forward reaction is 120 kJ mol-1. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic.
Answer the following questions about nitrogen, hydrogen, and ammonia. (a) In the boxes below, draw the complete Lewis electron-dot diagrams for N ... at 298 K according to the reaction represented below. N 2 (g) ... dot diagrams of nitrogen gas and ammonia. In part (b) the incorrect use of a mass-to-mass ratio with no mole-to-energy ratio did ...
Use the diagram to answer the questions below. Is the reaction endothermic or exothermic? What is the activation energy of the reaction? Show transcribed image text The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. Is the reaction endothermic or ...
Chemistry*12* Potential*Energy*Diagrams*Worksheet* Name:* Date:* Block:*! USE!THE!POTENTIAL*ENERGY*DIAGRAM!TO!ANSWER!THEQUESTIONS!BELOW:! 1.! Is!the!overall!reaction ...
Which energy diagram best reprsents the given reaction ?
Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which the reaction occurs?
Energy Diagram Practice Gap-fill exercise Fill in all the gaps, then press "Check" to check your answers. Use this energy diagram to answer these questions. 1. The enthalpy of the reactants of the reaction is about kilojoules. 2. The enthalpy of the products of the reaction is about kilojoules. 3.
This collection of web resources contains materials for the core units of the Saskatchewan Evergreen Curriculum for Chemistry 30. Links to each unit are found in the gray navigation bar near the top of each page · Each unit contains the following resource materials:
Question: D to answer Use the energy diagram for the reaction A the questions. How many transition states are there in the reaction? transition states: ...
Chemistry 12 Unit 1-Reaction Kinetics Worksheet 1-2 Potential Energy Diagrams Page 1 Chemistry 12 Worksheet 1-2 - Potential Energy Diagrams USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. Is the overall reaction as shown exothermic or endothermic? _____ 2. What is the activation energy for the forward reaction?
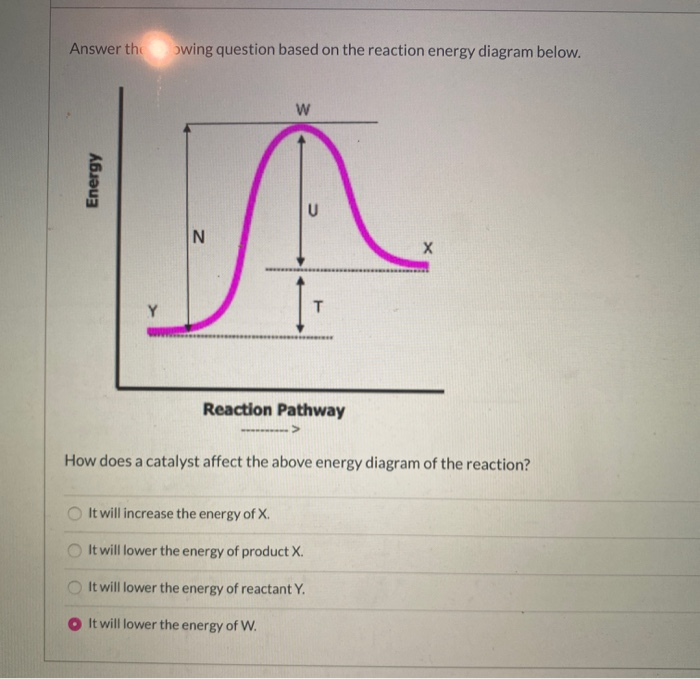
Activity Index · Thermochemistry and Energy Diagrams · Show all questions · The line that represents the activation energy (Ea) of this reaction is · Line A · Line B · Line C · Line D · Line E · The line that represents the heat of reaction (ΔH, or ΔE) of this reaction is
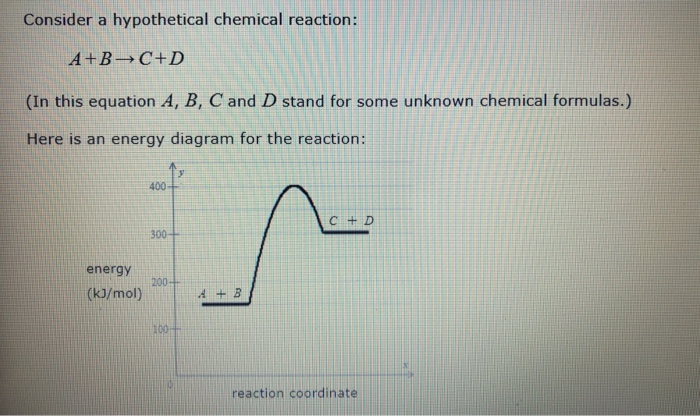
Here is an energy diagram for the reaction: Use the energy diagram to answer these questions. Can you determine the activation energy of the reverse reaction? C + D → A + B. All Chemistry Practice Problems Energy Diagram Practice Problems. See all problems in Energy Diagram. Frequently Asked Questions.
Use the following energy profile diagram to answer Questions (a) to (c) below. (a) From the diagram, determine the value of the; (i) enthalpy of the reaction (ii) activation energy of the reaction. (b) State whether the profile diagram is for an endothermic reaction or an exothermic reaction.
Ask any question and get an answer from our subject experts in as little as 2 hours.
Free practice questions for College Chemistry - Reaction Coordinate Diagrams. Includes full solutions and score reporting.
Answer to study the following reaction energy diagram: products energy Then answer the following questions about the chemical reac...
For a forward reaction, the activation energy is equal to the difference between the threshold energy and the energy level of the reactants. Once you identify the threshold energy and the energy level of the reactants, use a double arrowhead line to connect these two points on the potential energy diagram.
Use the energy diagram for the rearrangement reaction of methyl isonitrile to acetoni- trile to answer the following questions. 7. What kind of reaction is represented by this diagram, endothermic or exothermic? 8. What is the chemical structure identified at the top of the curve on the diagram? Cor«plc¥ 9. What does the symbol E represent? 10.
Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG, for the step A to B. kcal/mol. Calculate the overall energy change, ΔG°, for the process B to C. kcal/mol. Which step is faster, (a) A to B or (b) C to B. fullscreen Expand.
Transcribed image text: Use the energy diagram for the reaction A D to answer the questions How many transition states are there in the reaction?
Answer to Use the molecular orbital energy diagram below to answer the questions about bond order for the positive ion B2 Number o...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
O were to decompose via reaction 4, approximately how much energy would be released or absorbed? (A) 65 kJ of energy will be absorbed. (B) 65 kJ of energy will be released. ... Use the following diagram to answer questions 13-15. Reaction Coordinate Potential Energy 1 2 3 4
Use the diagram to answer the questions below.a. Is the reaction endothermic or exothermic?b. What is the activation energy of the reaction? FREE Expert Solution Show answer. 99% (496 ratings) FREE Expert Solution. Endothermic - absorbing energy. Exothermic - loss of energy.
25 E 20 é 15 C D 0 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, Î G , for the step A to B. Calculate the overall energy change, AGo, for the process B to C.
Transcribed image text: Use the energy diagram for the reaction AD to answer the questions. How many transition states are there in the reaction?


















0 Response to "44 use the energy diagram for the reaction to answer the questions."
Post a Comment