41 sn-pb phase diagram
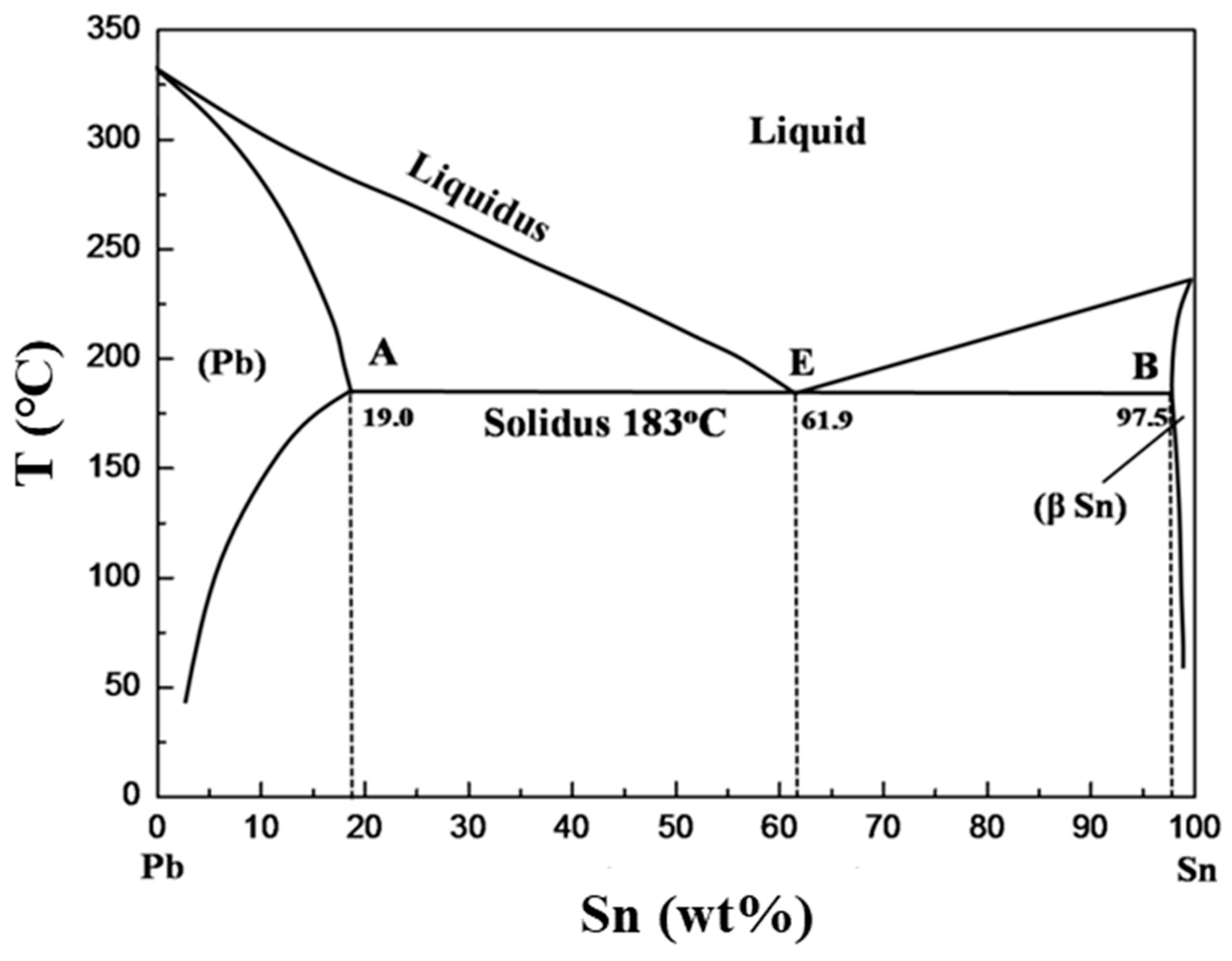
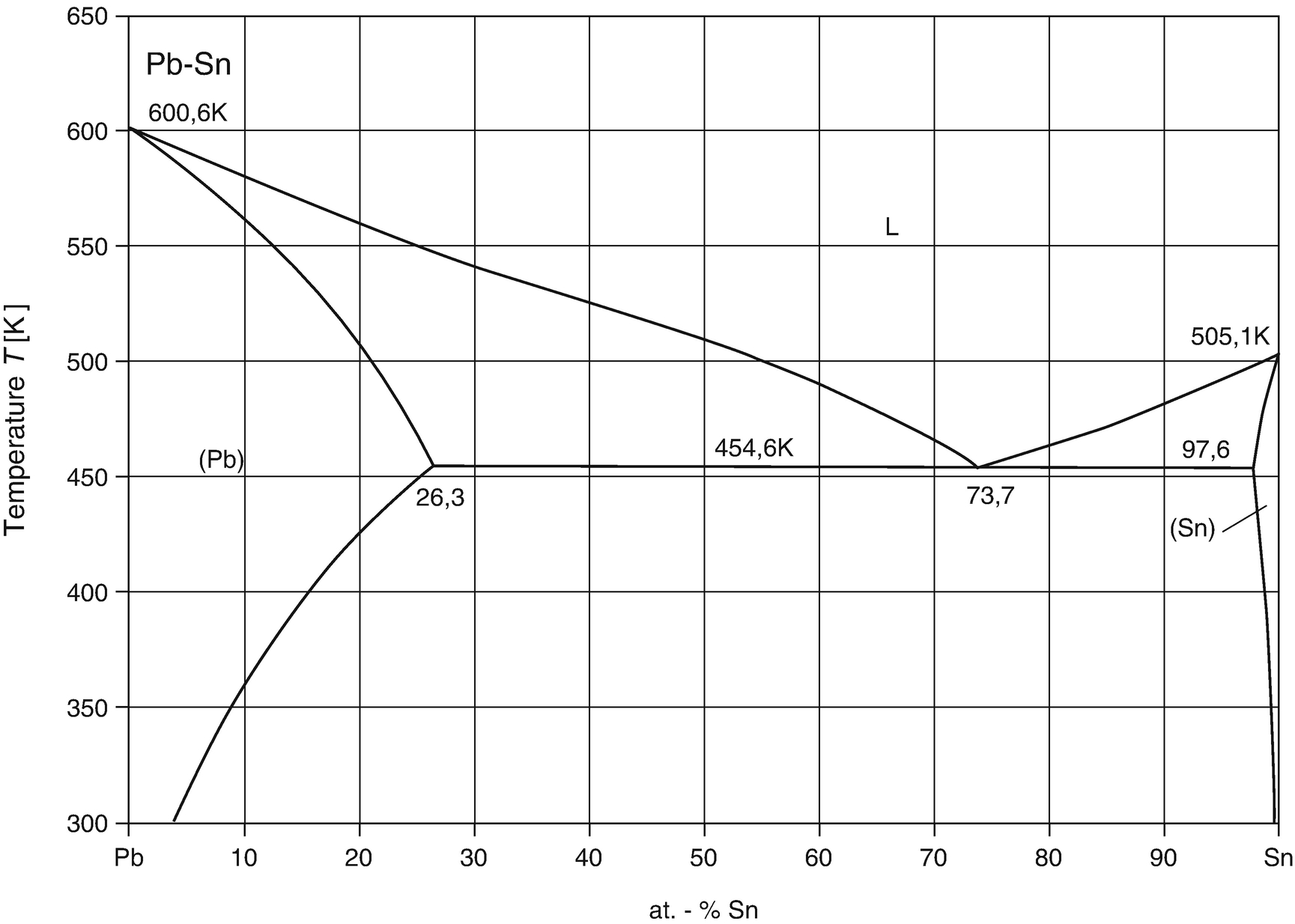
The Pb-Sn phase diagram calculated with the data from Ref. [ 38 ]. 3.3. The solution phases The bct (β-Sn, labeled Sn in consequent text) and diamond (α-Sn) phases were modeled as substitutional solid solutions. The LIQUID phase was also modeled using a substitutional model with one sublattice. Aug 01, 2021 · More recently, it was demonstrated that the antibacterial properties were mainly controlled by the precipitation of Ag-or Cu-containing phase rather than the Ag or Cu ion release . However, there are also some drawbacks of antibacterial metals and alloys: 1) Mechanical properties and corrosion property.
Oct 01, 2021 · High-entropy ceramics with five or more cations have recently attracted significant attention due to their superior properties for various structural …
Sn-pb phase diagram
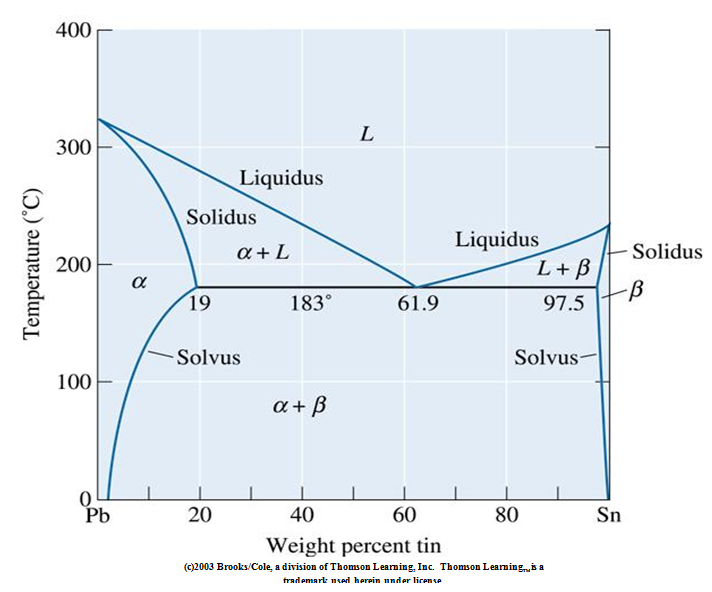
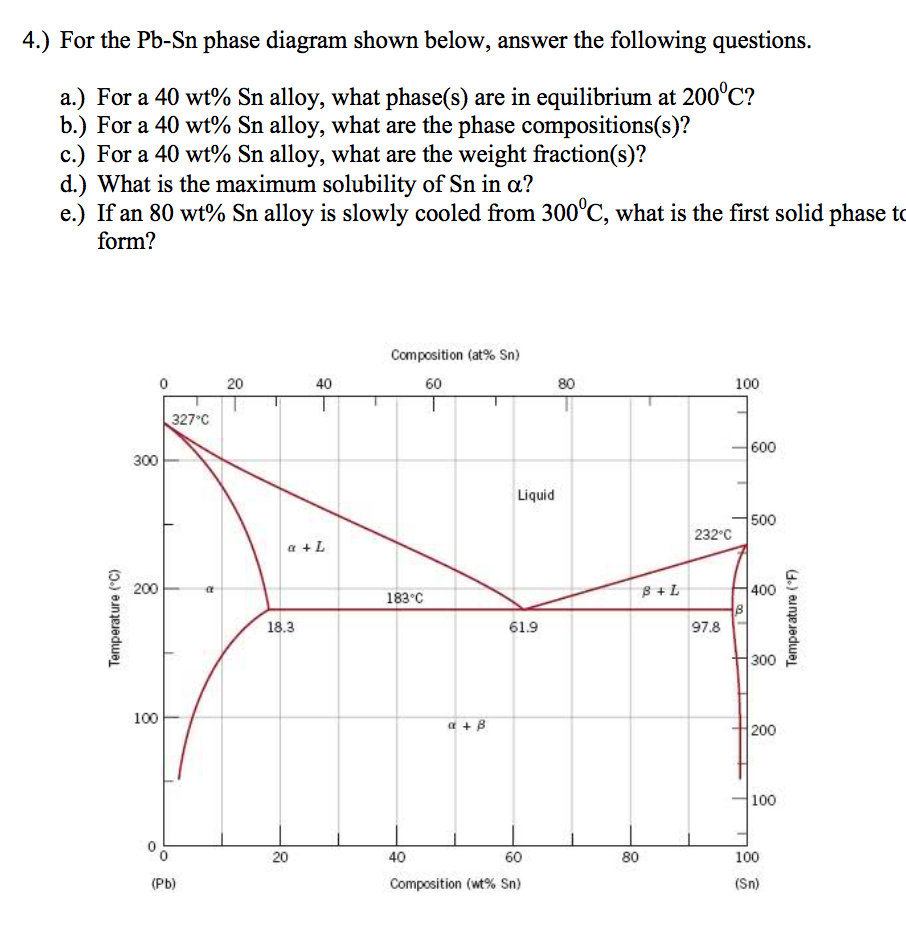
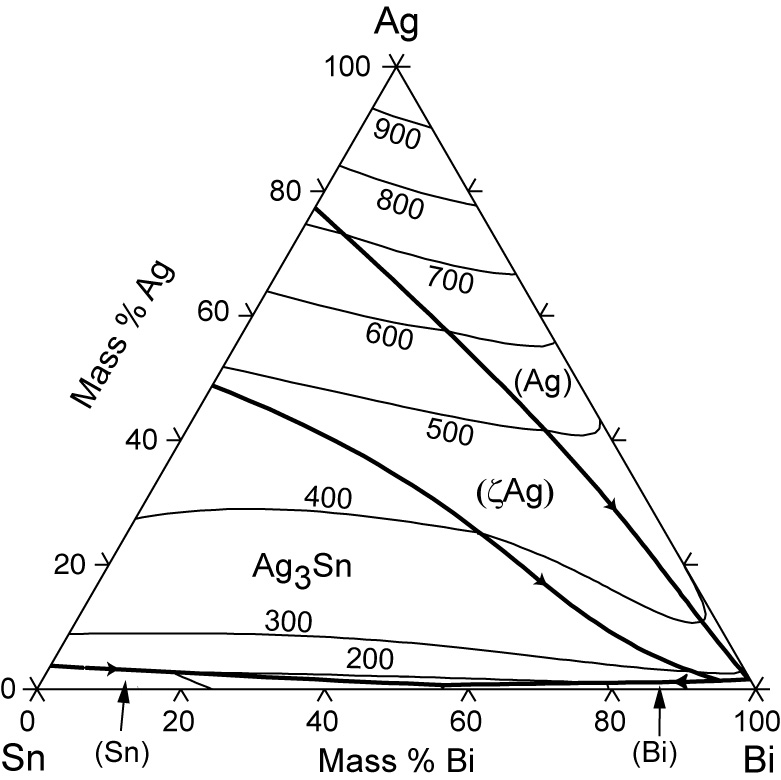
That portion of the Pb-Sn phase diagram (Figure 9.8) that pertains to this problem is shown below; the point labeled "H" represents the 85.1 wt% Sn-14.9 wt% Pb composition at 200°C. As may be noted, point H lies within the β + Lphase field. SGTE Alloy Phase Diagrams. Click on a system to display the phase diagram. The study of ternary Ag-Pb-Sn phase diagram is important e.g. for the soldering industry. Pb-Sn alloys have been used as solders for a long time [1,2]. Even though the eutectic Pb-Sn has been prohibited from use in electronic products since 2006 [3], Pb-Sn alloys with Pb content higher than 85% are still in use [4].
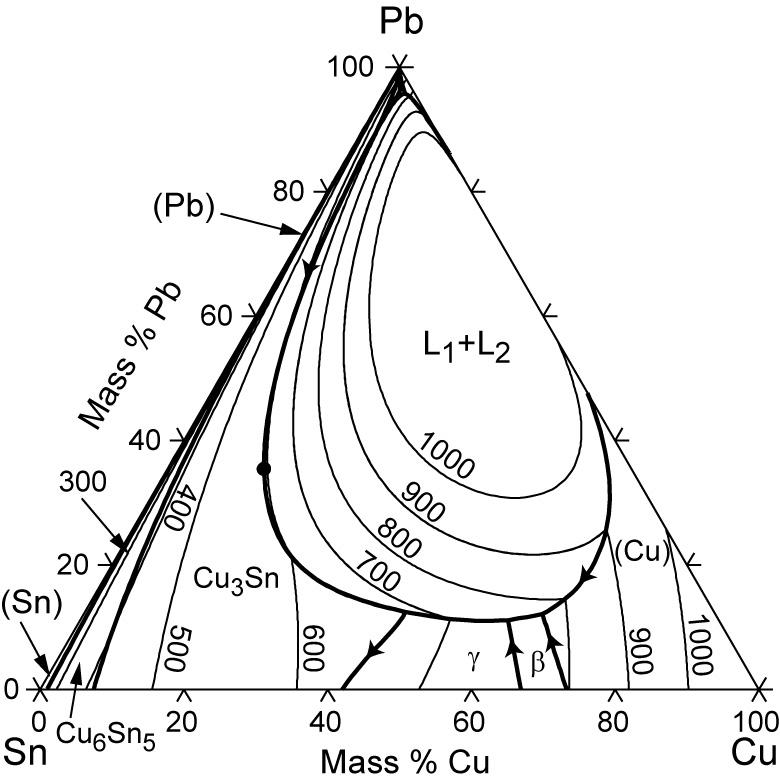
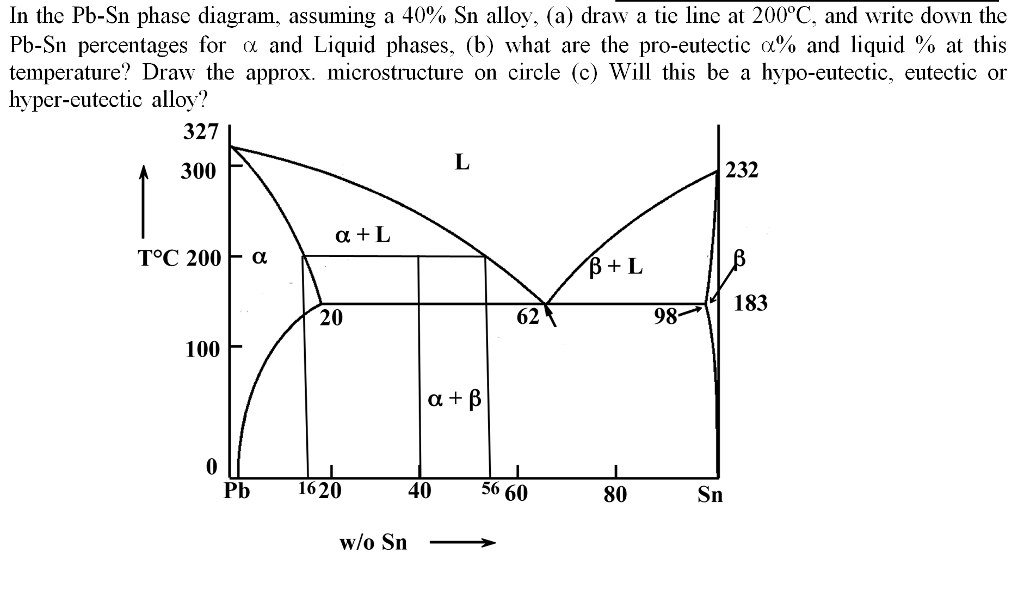
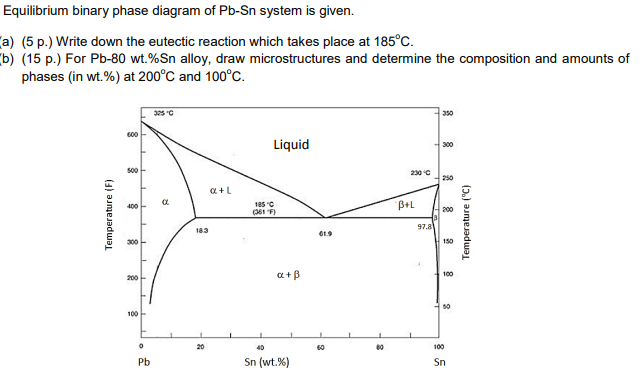
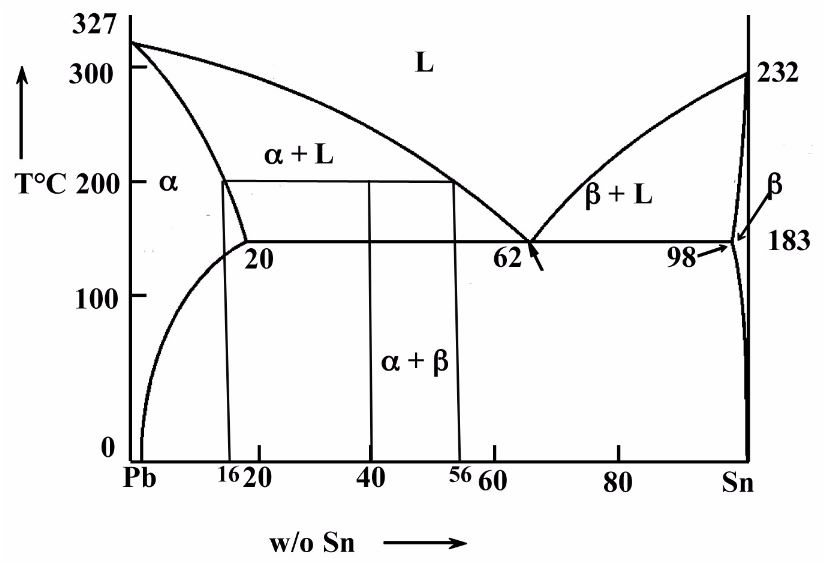
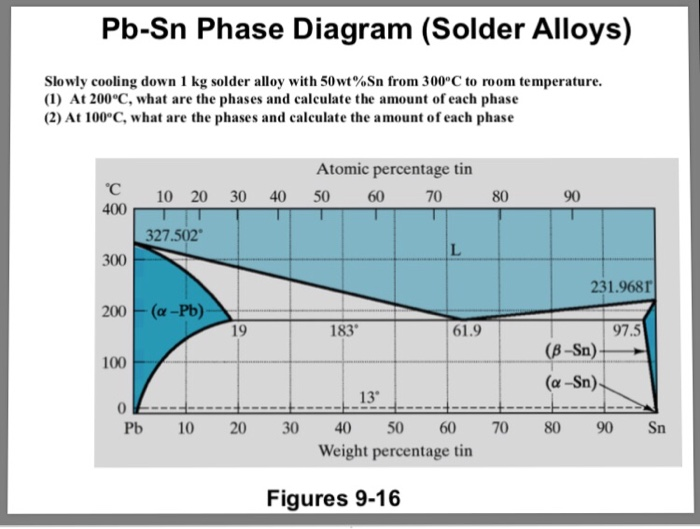
Sn-pb phase diagram. In the Pb-Sn phase diagram below, there are 6 phase fields: three shaded purple and three shaded white. The three purple phases fields are single phase regions, regions in which only one phase exists, and the white phase fields are two-phase regions. Phase Analysis from Sn-Pb Phase Diagram 3. Question: For a 40-60 Pb-Sn solder, find ; a) Phase present, Composition of phases and Weight fraction at 200˚C b) Phase present, Composition of phases and Weight fraction at 100˚C 4. Phase Diagram: 5. Thermal analysis of Sn-Pb alloys, construction of phase diagram ... eutectic composition, eutectic temperature, phase diagram, intensive and extensive ...10 pages Section-Editor: Hiroaki Okamoto (Phase Diagrams) Cite this content Pierre Villars (Chief Editor), PAULING FILE in: Inorganic Solid Phases, SpringerMaterials (online database), Springer, Heidelberg (ed.) SpringerMaterials Pb-Sn Binary Phase Diagram 0-40 at.%
Figure 1 shows the Pb-Sn phase diagram and the composition for present experiments, in which it is very clear that the eutectic temperature is 183 • C, and the liquid-solid transition temperature... suggested as possible alternatives to Pb-Sn solders. 19 Figure 6 shows the effect of the addition of Ag, Bi, Sb, or Zn on the phase constitution of Sn-20mass%In alloys. a Fig. 3. Isothermal section diagrams of the Sn-In-Bi system at (a) 100°C and (b) 200°C. a Fig. 4. Isothermal section diagrams of the Sn-In-Sb system at (a) 100°C and (b) 200 ... Jul 13, 2021 · However, it has been widely observed that Sn-based (including Sn–Pb) perovskite materials show inferior light-emitting properties (Supplementary Table 1) 9,11,13,15,16,20,21,22,23,24 compared to ... High-entropy alloys (HEAs) are alloys that are formed by mixing equal or relatively large proportions of (usually) five or more elements.Prior to the synthesis of these substances, typical metal alloys comprised one or two major components with smaller amounts of other elements.
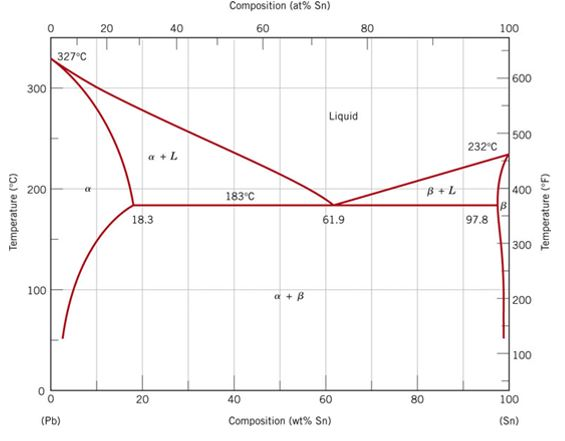
Pb-Sn phase diagram β phase: solid solution of Pb in tetragonal Sn α phase: solid solution of Sn in fcc Pb Liquid Pb (Fcc) Sn (Tetra) 0 50 100 150 200 250 300 350 0 10 20 30 40 50 60 70 80 90 100 T emperature Wt% The Pb-Sn system is characteristic of a valley in the middle. Such system is known as the Eutectic system. The central point is the ... This problem has been solved! At 100 C, what is the maximum solubility (a) of Pb in Sn? (b) of Sn in Pb? The lead-tin phase diagram is shown below. Round to the nearest whole number. Who are the experts? Experts are tested by Chegg as specialists in their subject area. The binary Pb-Sn phase diagram has been studied for over 100 years and is a classic eutectic. Lead (Pb) has an atomic number of 82 and a high density. Its crystal structure is face-centered cubic (fcc). At 50 C, 2% Sn is soluble in Pb and at the eutectic temperature, 183 C, the maximum solubility of Sn in -Pb is 19%. Sn-Bi、Sn-Zn、Sn-PbなどではIMCを形成せず、共晶となる。 Sn-Bi系 共晶点は58Biで139℃。 Sn-Zn系 共晶点は9Znで199℃。 Sn-Pb系 *Snへの固溶 →固体Snへの固溶
The Attempt at a Solution. It is probably an easy question, but I thought it is a good idea to consult first. a) At 183 C, the first liquid phase forms. b) We can draw a tie line and the point intersects with the liquidus line, gives us the composition of liquid. It is 61.9 wt % Sn. c) It is around 250 C. Because phase diagram is on liquidus line.
β phase. Thus, the phase compositions are as follows: Cα = 16 wt% Sn-84 wt% Pb Cβ = 97 wt% Sn-3 wt% Pb (c) The Ag-Cu phase diagram (Figure 9.7) is shown below; the point labeled "C" represents the 55 wt % Ag-45 wt% Cu composition at 900 °C. As may be noted, point C lies within the Liquid phase field.
We will use the Pb-Sn phase diagram as an example. Pb-Sn alloys are used as common solders. A eutectic reaction is one in which the liquid phase solidifies to produce two solid phases. eg in the Pb-Sn eutectic system, Liquid reacts to form α + , ie L α + . Eutectic systems are relatively simple and common.
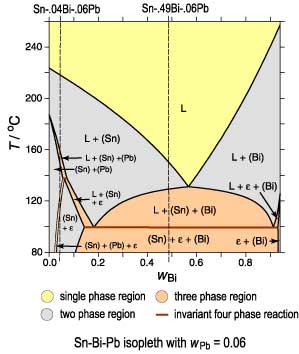
The (Sn) phase is the primary phase in all cases. Under Scheil conditions the alloy Sn-.04Bi-.06Pb encounters the L -> (Sn) + (Pb) monovariant eutectic where both phases form simultaneously from the liquid phase. The path encounters then the four phase reaction, L + (Pb) -> (Sn) + epsilon.
phase diagrams in this chapter to help you visualize the various microstructures that form during solidification. The single-phase regions of the Pb-Sn ...6 pages
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Alloy Phase Diagrams 9 (1988) 144-152. H. Ohtani, K. Okuda and K. Ishida, J. Phase Equilibria 16 (1995) 416-429. Calculated Invariant Equilibria. Reaction. Phase. Mass % Pb. Mass % Sn. L -> (Pb) + (Sn) 182.2 o C.
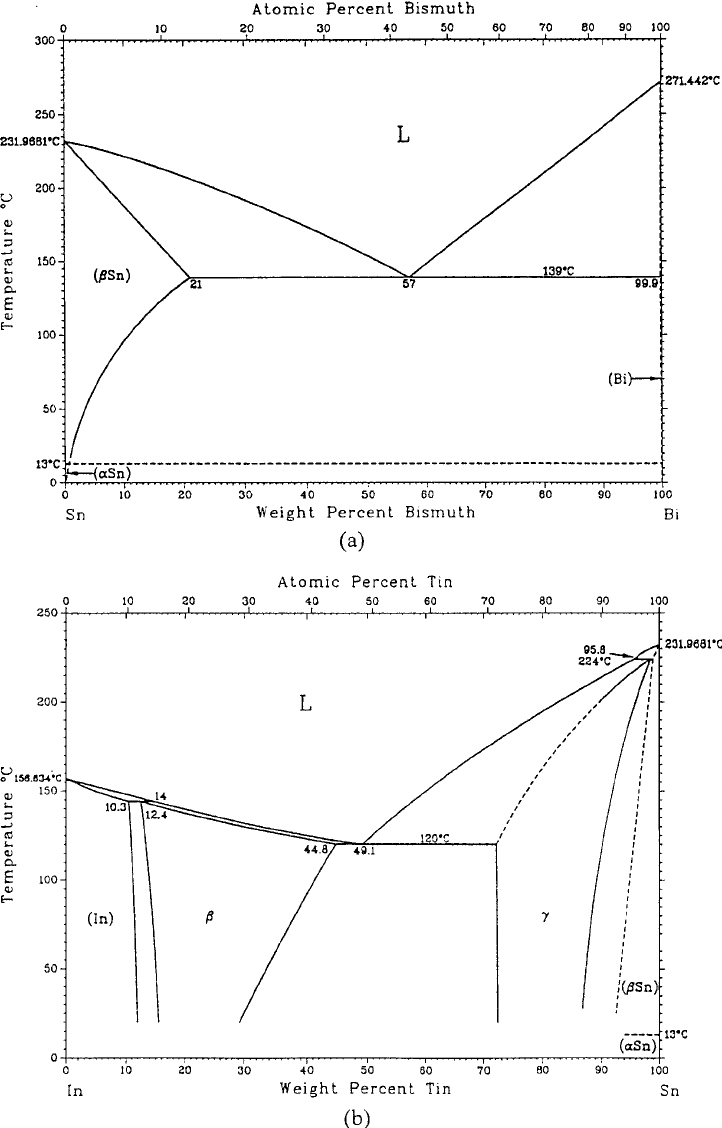
Phase Diagrams & Computational Thermodynamics. Bi-Pb-Sn System. Calculated Liquidus Projection: Status of the thermodynamic description: S.W. Yoon and H.M. Lee, CALPHAD 22 (1998) 167-178 ... (Bi,Pb,Sn) 1 * Major species are printed bold face. Materials Science and Engineering Division ...
Example using the Pb-Sn Phase Diagram. Consider a 40 wt% Sn-60 wt% Pb alloy on the lead-tin phase diagram. Part 1: At 183.1 degrees C, just above the eutectic line, a) what phase(s) is (are) present? b) what is (are) the compositions of the phase(s)? c) what is the relative amount of each phase present, in mass fraction?
#modimechanicalengineeringtutorials, #mechanicalmagicmechanicallearningtutorials,Welcome to My YouTube Channel MODI MECHANICAL ENGINEERING TUTORIALS.This ch...
Pb-Sn Eutectic at 960C M as s F r a t i o n o f B i M s s F r c t o n o f P b Bi Mass Fraction of Sn Sn t c / 0 C prism is a two-component temperature-composition phase diagram with Pb Triple Eutectic 3-Dimensional Depiction of Temperature-Composition Phase Diagram of Bismuth, Tin, and Lead at 1atm. The diagram has been simplified by
The composition of the eutectic phase pf Sn-Pb alloy can be turned into liquid directly from the solid phase, without going through the solid+liquid phase. Sn-Pb alloys are generally used in electrical and electronic components in today's technology. Conclusion. The assessment of Sn-Pb binary phase diagram is very easy like above.
This video explains the Pb-Sn phase diagramFor further reading: https://www.physicsforums.com/threads/sn-pb-phase-diagram.281790/
Sugar/Water Phase Diagram S u g a r T e m p e r a t u r e ... the Pb-Sn system during solidification at the eutectic composition. Compositions of α and β phases are very different. Solidification involves redistribution of Pb and Sn atoms by atomic diffusion. Pb-rich
The Pb-Sn phase diagrams with and without current stressing clearly demonstrate the change in the phase stabilities of Pb-Sn solders under current stressing. Introduction Soldering is one of the...
In the Pb-Sn phase diagram on the right, an alloy contains 64 wt% proeutectic α and 36 wt% of eutectic α+β at 180oC -ΔT, find the average composition of this alloy A. 34.6% Sn B. 65.4 % Sn C. 64 % Sn D. 27.3% Sn Answer: Since the alloy contains proeutectic , so the average composition of this alloy must vary between 19.2%( ) and ...
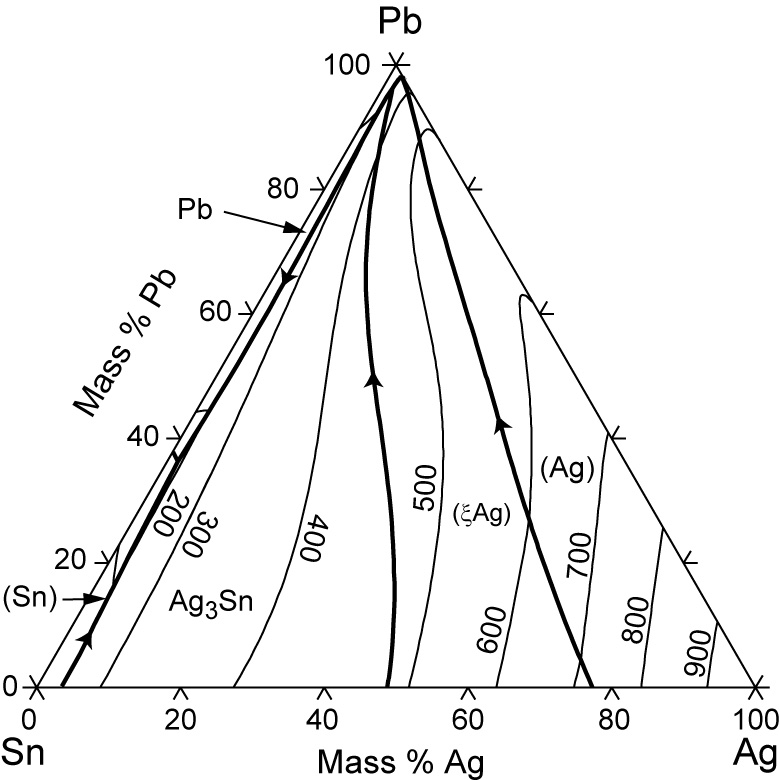
The study of ternary Ag-Pb-Sn phase diagram is important e.g. for the soldering industry. Pb-Sn alloys have been used as solders for a long time [1,2]. Even though the eutectic Pb-Sn has been prohibited from use in electronic products since 2006 [3], Pb-Sn alloys with Pb content higher than 85% are still in use [4].
SGTE Alloy Phase Diagrams. Click on a system to display the phase diagram.
That portion of the Pb-Sn phase diagram (Figure 9.8) that pertains to this problem is shown below; the point labeled "H" represents the 85.1 wt% Sn-14.9 wt% Pb composition at 200°C. As may be noted, point H lies within the β + Lphase field.



















0 Response to "41 sn-pb phase diagram"
Post a Comment