42 adiabatic process pv diagram
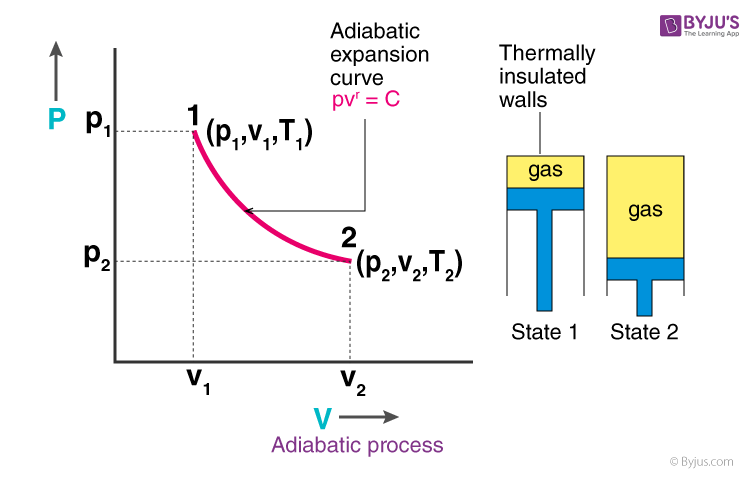
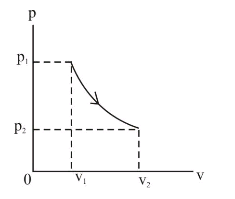
This equation is the condition that must be obeyed by an ideal gas in a quasi-static adiabatic process. For example, if an ideal gas makes a quasi-static adiabatic transition from a state with pressure and volume and to a state with and then it must be true that . The adiabatic condition of can be written in terms of other pairs of thermodynamic variables by combining it with the ideal gas law. So on a PV diagram, an isothermal process is gonna look something like this, it's gonna curve like 1/x and it can be an isothermal expansion if volume increases or an isothermal compression if volume decreases. So the actual shape of the line drawn on a PV diagram for an isothermal process is sometimes called an isotherm and they look like that.
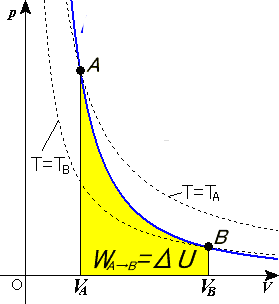
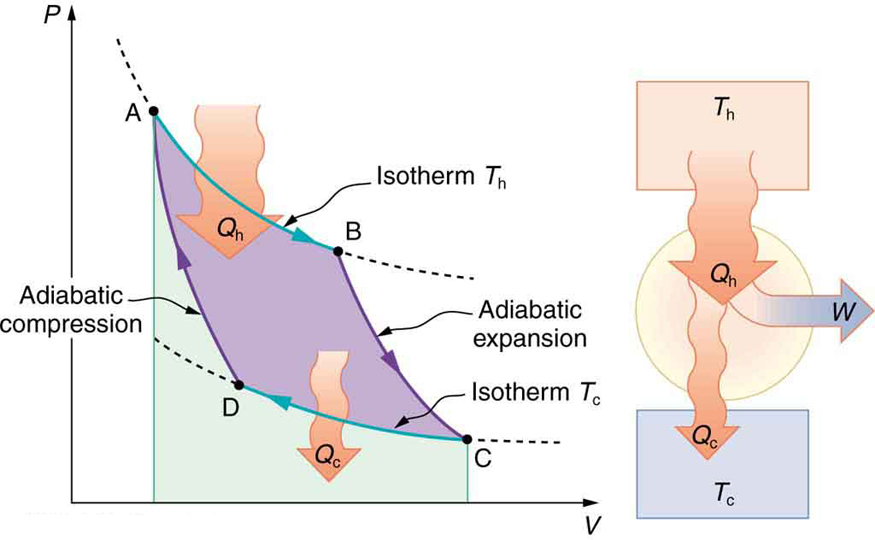
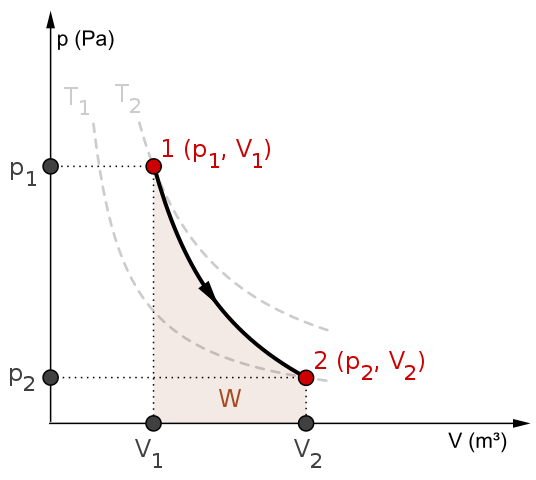
During an adiabatic process no heat is transferred to the gas, but the temperature, pressure, and volume of the gas change as shown by the dashed line. As described on the work slide, the area under a process curve on a p-V diagram is equal to the work performed by a gas during the process. On the right of the figure we have plotted the ...
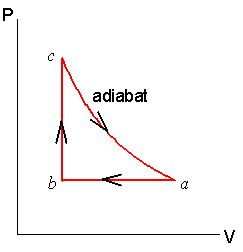
Adiabatic process pv diagram
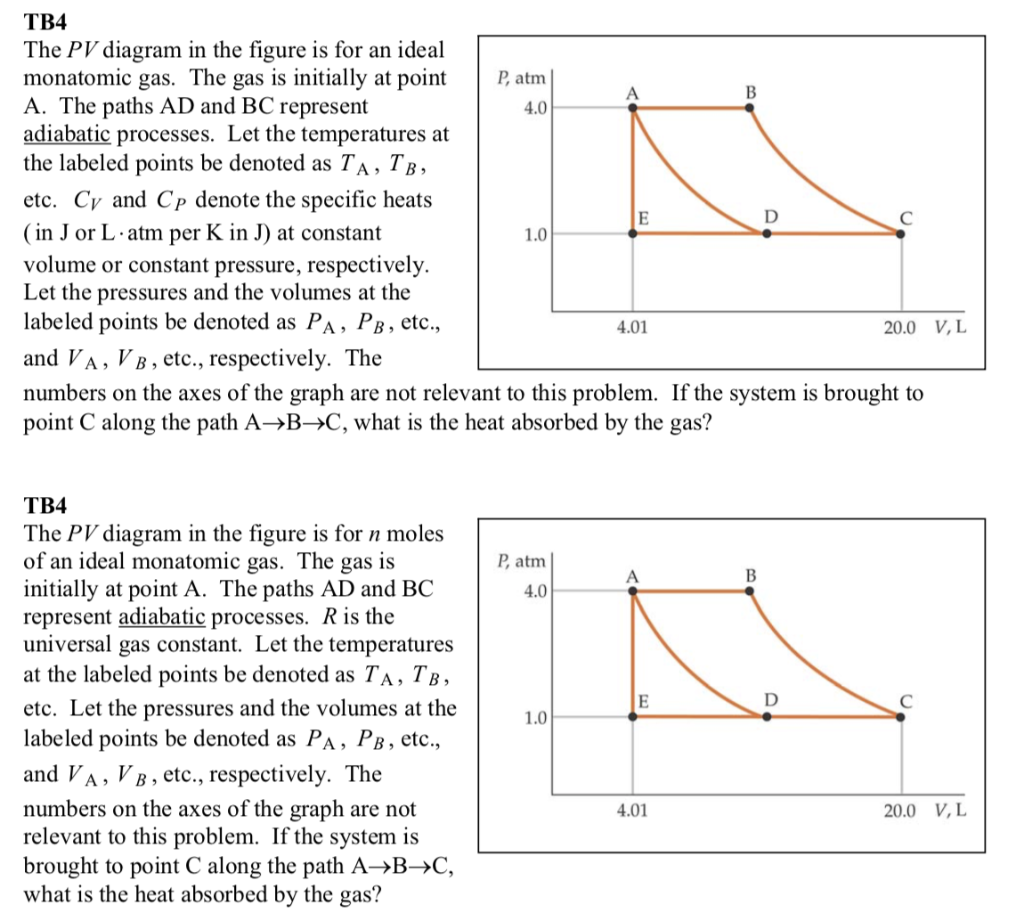
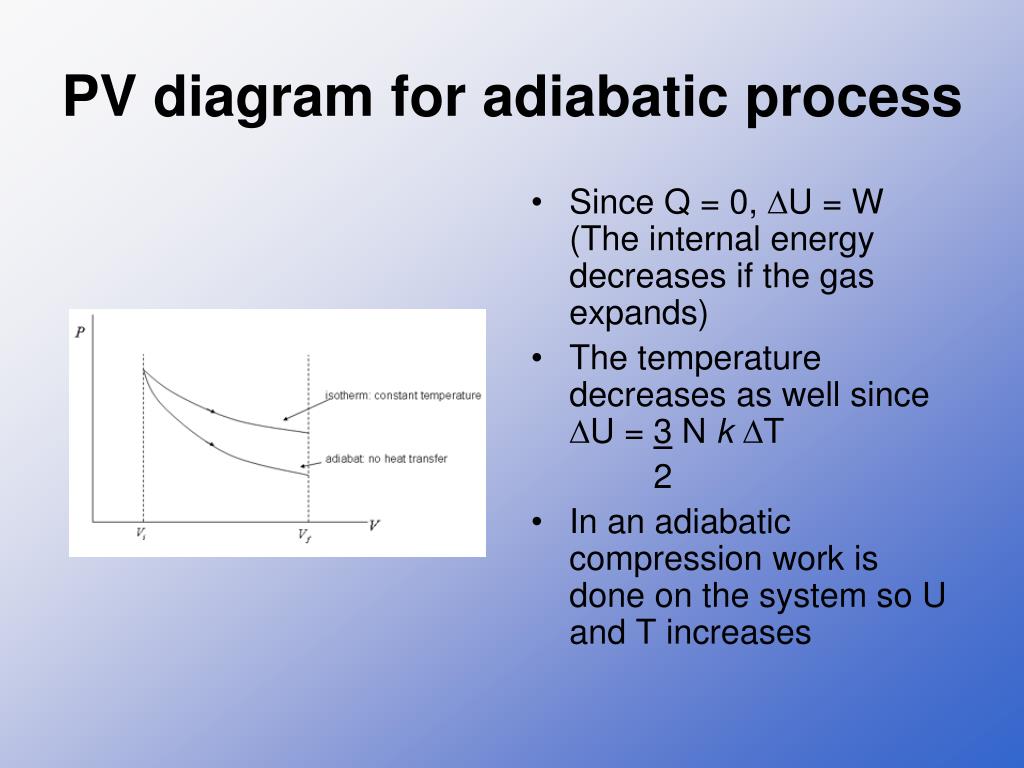
An adiabatic process is one in which no heat is gained or lost by the system. The first law of thermodynamics with Q=0 shows that all the change in internal ... pV γ pVγ 1 constant 2 2 1 1 1 = = T Vγ− T V γ− pV =nRT During an adiabatic expansion process, the reduction of the internal energy is used by the system to do work on the environment. During an adiabatic compression process, the environment does work on the system and increases the internal energy. Ideal gas: adiabatic process (contd) When we subject the gas to these thermodynamics processes, the pressure and volume of the gas can change. A convenient way to visualize these changes in the pressure and volume is by using a Pressure Volume diagram or PV diagram for short. Each point on a PV diagram corresponds to a different state of the gas.
Adiabatic process pv diagram. In thermodynamics, an isentropic process is an idealized thermodynamic process that is both adiabatic and reversible. The work transfers of the system are frictionless, and there is no net transfer of heat or matter. Such an idealized process is useful in engineering as a model of and basis of comparison for real processes. This is idealized as reversible processes do not occur … During an adiabatic process no heat is transferred to the gas, but the temperature, pressure, and volume of the gas change as shown by the dashed line. As ... The adiabatic process is a thermodynamic process in which there is no heat transfer from in or out of the system. For an ideal gas, an adiabatic process is a reversible process with constant entropy. The mathematical representation of the adiabatic process is ΔQ=0 On a p-V diagram, an adiabatic process occurs along a line (called an adiabat) that has the equation p = constant / Vκ. Adiabatic Curve - Adiabat.
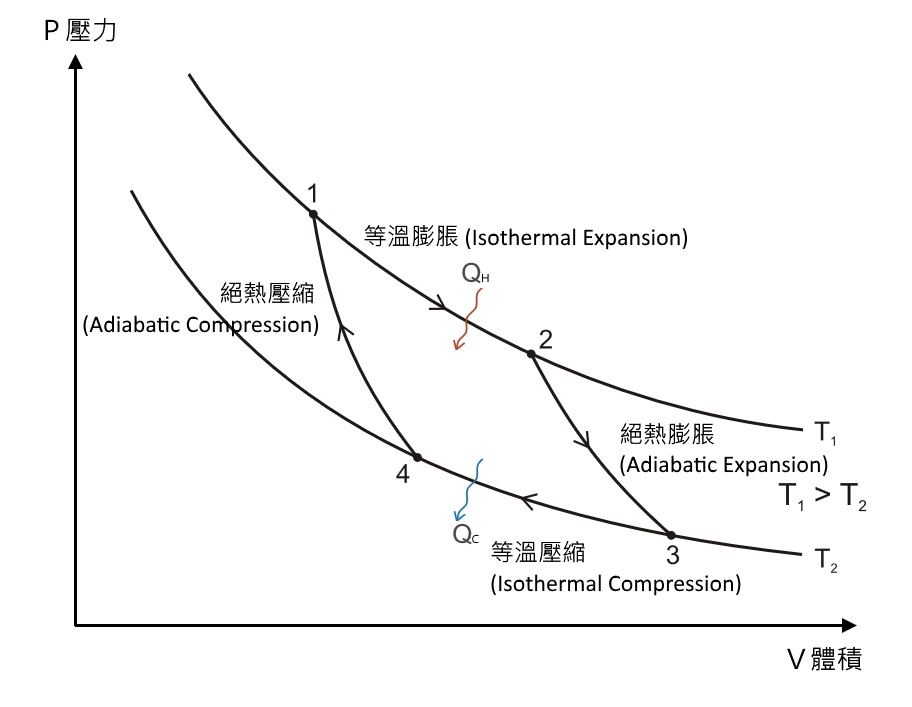
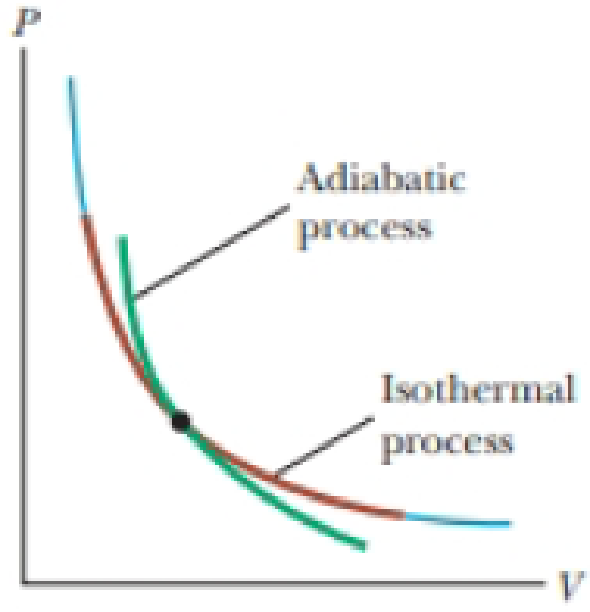
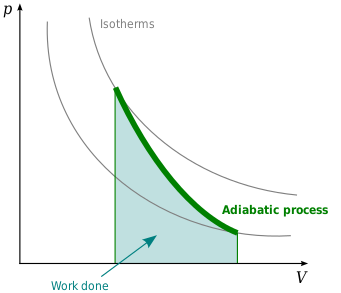
Nov 8, 2021 — A reversible adiabatic expansion of an ideal gas is represented on the pV diagram of Figure 3.7.1. The slope of the curve at any point is. Answer (1 of 2): On a PV diagram, only adiabatic and isotherm processes have asymptotic shapes. An example case for the curves for the two processes is shown here: It is not possible to just look at the shape of one curve and predict if it adiabatic or isotherm, because they look very similar. ... However, the former exist only as theoretical tools to study the latter. Thus, reversible adiabatic processes involve ideal gases, and lack friction and any other eventuality that causes heat transfer between the system and its surroundings. Consider for example the PV diagram for the reversible adiabatic process above. Process 1-2: Reversible Adiabatic Compression Process: The cylinder contains full of air which is entered through the inlet port as we studied above. Here P1, V1, and T1 are the corresponding Pressure, Volume, and Temperature. After the adiabatic compression process in which entropy remains constant and the air is compressed by the piston.
1. This question does not show any research effort; it is unclear or not useful. Bookmark this question. Show activity on this post. In non isolated systems where there is no adiabatic process, P V is constant. But the graph gets steeper in adiabatic process because of the γ over the V. Why is it there in adiabatic processes and why only over ... May 22, 2019 · Adiabatic Process. An adiabatic process is a thermodynamic process, in which there is no heat transfer into or out of the system (Q = 0). The system can be considered to be perfectly insulated.In an adiabatic process, energy is transferred only as work. The assumption of no heat transfer is very important, since we can use the adiabatic approximation only in … The PV diagram for an adiabatic process show a special result. • An adiabatic process looks very much like an isothermal process, but it drops off to a lower point. This means it is on a different isotherm. Remember, isotherms are just lines that show where the temperature stays constant. As we've already discussed, in the adiabatic here , we can conclude that the slope of PV diagram of Polytropic process is negative and greater than isothermal process but less than Adiabatic process. Because , 1 < N < ¥ Therefore the P-V diagram of Polytropic process will be like :
Visit us (http://www.khanacademy.org/science/healthcare-and-medicine) for health and medicine content or (http://www.khanacademy.org/test-prep/mcat) for MCAT...
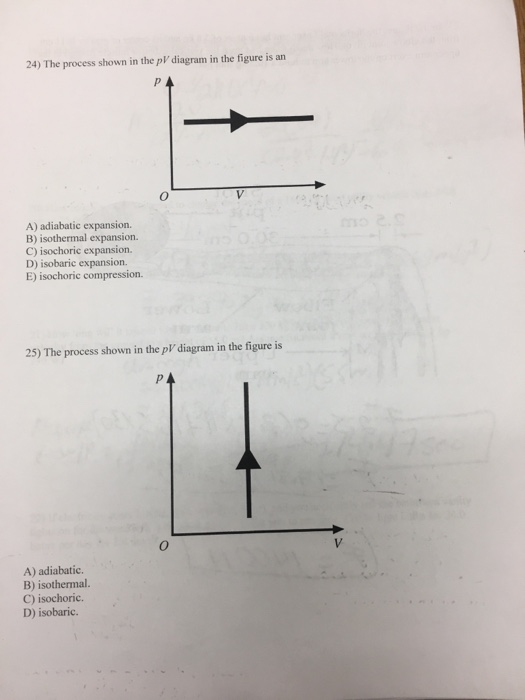
This physics video tutorial provides a basic introduction into PV diagrams. It explains how to calculate the work done by a gas for an isobaric process, iso...
Isothermal process on p-V, T-V, and p-T diagrams ... ideal gas law: pV = nRT Consider the p-V diagram below in which the system evolves from a ... Adiabatic processes reversible a = (p 1, V 1, T 1) b = (p 2, V 2, T 2) a p p 1 p 2 V 1 V 2 b V T 1 T 2 isotherms adiabat pV ...
Adiabatic Vs Isothermal. In a thermodynamics system, the two main processes associated are adiabatic or isothermal. It is regarded as the erstwhile when the transformation (variations in temperature) is fast acceptably that no heat was significantly transferred between the outside environment and the system.
While a truly adiabatic process is not possible, near adiabatic conditions can be achieved by reducing the volume rapidly or using a very well insulated container. When an ideal gas undergoes an adiabatic expansion or compression, the adiabatic equation can be used: $$ pV^{\gamma} = \text{constant} $$
The paths look somewhat similar on the P-V diagram, but you should notice clear differences. Note that an isothermal process has no change in temperature, so the change in internal energy is zero, but in an adiabatic process the heat transferred is zero. Note that for each press of a button, the volume will change by 1 liter, unless that ...
The mathematical equation for an ideal gas undergoing a reversible (i.e., no entropy generation) adiabatic process can be represented by the polytropic process equation =, where P is pressure, V is volume, and for this case n = γ, where = = +, C P being the specific heat for constant pressure, C V being the specific heat for constant volume, γ is the adiabatic index, and f is the number of ...
Sep 08, 2019 · Process 4-1 Isentropic Compression. Now again the insulated cap I.C. is brought in contact with the bottom of the cylinder B, and the air is allowed to be compressed adiabatically. The adiabatic compression is represented by the curve 4-1 on p-v and T-s diagram. The temperature of the air increases from T4 to T1. Since no heat is absorbed or ...
A: cross-sectional area of a piston. dV= Adx: increase in the volume of gas. P 1, V 1, T 1: Initial state of Gas. P 2, V 2, T 2: Final state of Gas. Total Work done of the gas is given by: Also, we know that P 1 V 1 and P 2 V 2 are equal to nRT 1 and nRT 2 respectively. So the equation for work done in the adiabatic process is also given by.
adiabatic no heat exchange with the environment; adiabatic has a complex greek origin that means "not+through+go": α + ∆ια + βατός [a + dia + vatos] examples: "fast" processes, forcing air out through pursed lips, bicycle tire pump; PV diagram is a "steep hyperbola"
The combustion process inside a car engine is essentially adiabatic for this reason. An isobaric process is a process that occurs at constant pressure. We then have W = P(V 2 - V 1). If the pressure of an ideal gas is kept constant, then the temperature must increase as the gas expands. (PV/T = constant.)
In an isothermal process the initial room temperature is constant. W = 1 × 8.3 × 300 × ln(2) = 1.7kJ (b) Comparing all three processes, we see that the work done in the isobaric process is the greatest, and work done in the adiabatic process is the least. (c) The PV diagram is shown in the Figure.
This equation is the condition that must be obeyed by an ideal gas in a quasi-static adiabatic process. For example, if an ideal gas makes a quasi-static adiabatic transition from a state with pressure and volume p 1 p 1 and V 1 V 1 to a state with p 2 p 2 and V 2, V 2, then it must be true that p 1 V 1 γ = p 2 V 2 γ. p 1 V 1 γ = p 2 V 2 γ.
An adiabatic process is a thermodynamic change whereby no heat is exchanged between a system and its surroundings ( q. = 0). For an ideal gas undergoing an adiabatic process, the first law of thermodynamics may be written, from Eq. (15), (16) d ln T − R c p d ln p = 0. Denoting R / cp by κ and integrating this from a given pressure and ...
Process 3-4: Reversible Adiabatic Expansion. It is also called a reversible adiabatic expansion process. In this process, at point 3, the supply of heat is stopped and this point is known as Point of Cut off. Later, the air expands to the conditions of P4, V4 and T4 respectively corresponding to point 4.
Solved Examples Question 1 A gas ($\gamma =1.4$) of 2m 3 Volume and at a pressure of $4 \times 10^5 N/m^2$ is compressed adiabatically to a volume .5 m 3.Find its new pressure. Calculate the work done in the process? Given $4^{1.4} =6.96$ Solution
Adiabatic Process A thermodynamic process in which there is no heat into or out of a system is called an adiabatic process. To perform an ideal adiabatic process it is necessary, that the system be surrounded by a perfect heat insulator. If a compression or expansion of a gas takes place in a short time, it would be nearly
On a pV diagram, the process occurs along a line called isothermal curve or an isotherm. This curve has the equation p = constant / V. It can be derived from ideal gas law. In an ideal gas, molecules have no volume and do not interact. According to the ideal gas law, pressure varies linearly with temperature and quantity, and inversely with volume.
This property of PV diagrams is very useful and broadly applicable: the work done on or by a system in going from one state to another equals the area under the curve on a PV diagram. Figure 5. A graph of pressure versus volume for a constant-pressure, or isobaric, process, such as the one shown in Figure 4.
The PV diagram for an adiabatic process is also called adiabat Note that the PV diagram for isothermal (Figure 8.25) and adiabatic (Figure 8.30) processes look similar. But actually the adiabatic curve is steeper than isothermal curve. We can also rewrite the equation (8.35) in terms of T and V.
When we subject the gas to these thermodynamics processes, the pressure and volume of the gas can change. A convenient way to visualize these changes in the pressure and volume is by using a Pressure Volume diagram or PV diagram for short. Each point on a PV diagram corresponds to a different state of the gas.
pV γ pVγ 1 constant 2 2 1 1 1 = = T Vγ− T V γ− pV =nRT During an adiabatic expansion process, the reduction of the internal energy is used by the system to do work on the environment. During an adiabatic compression process, the environment does work on the system and increases the internal energy. Ideal gas: adiabatic process (contd)
An adiabatic process is one in which no heat is gained or lost by the system. The first law of thermodynamics with Q=0 shows that all the change in internal ...























0 Response to "42 adiabatic process pv diagram"
Post a Comment