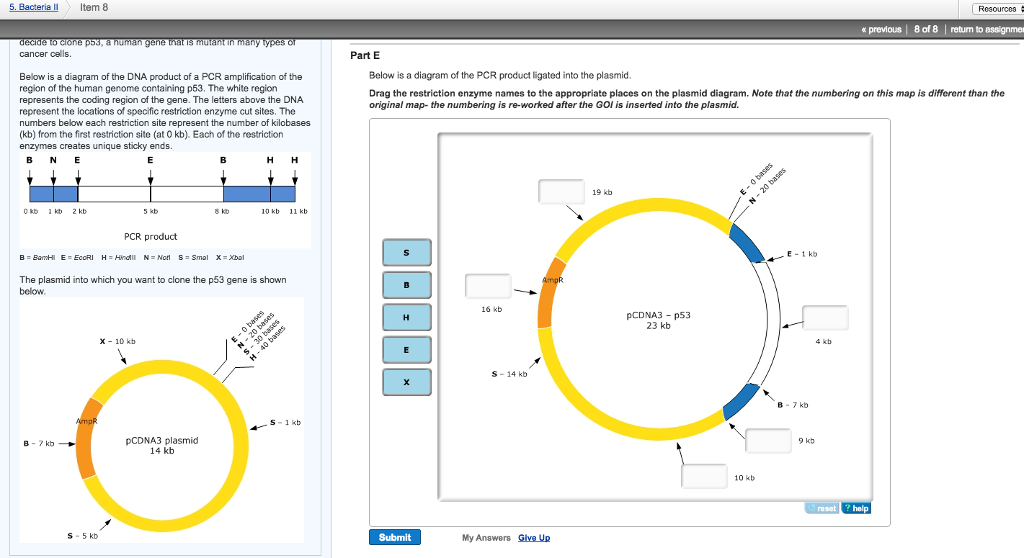
42 below is a diagram of the pcr product ligated into the plasmid.
26.12.2021 · 1 INTRODUCTION. Rice (Oryza sativa) is a major staple crop in the world.Bacterial blight caused by Xanthomonas oryzae pv.oryzae (Xoo) is one of the major diseases threatening rice production (Joshi et al., 2020).Xoo enters the rice leaf through wounds or hydathodes at the leaf tip and margin, then moves to and spreads through the xylem vessels (Mew et al., 1993). Suppose you find that the harE gene is in the plasmid, but now you want a restriction map of the recombinant plasmid. You take three individual samples of the plasmid and digest one sample with EcoRI, the second sample with HindIII, and the third sample with both EcoRI and HindIII. Then you run the digested DNA on a gel to see the fragments.
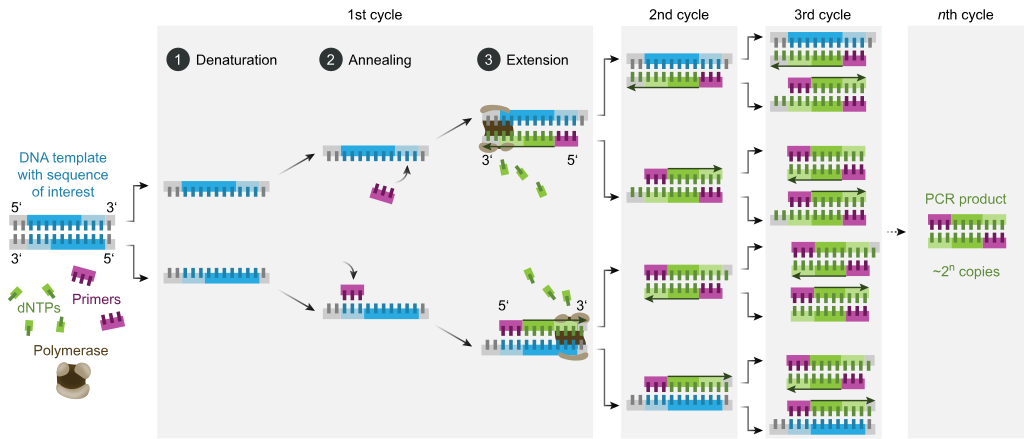
Ans. PCR stands for polymerase chain reaction.It is a technique for amplification of gene of interest or to obtain multiple copies of DNA of interest. The PCR requires primers, taq polymerase, target sequence, DNA sample &deoxyribonucleotides. PCR includes number of cycles for amplifying DNA of interest invitro. Each cycle has three steps :-
Below is a diagram of the pcr product ligated into the plasmid.
The PCR products obtained for P. atlantica with each primer set were ligated into the plasmid pCR2.1 and sequenced (see Methods and Materials). The primary products for both primer sets had the IS 492 right- and left-end sequences separated by a 5-bp junction sequence (Fig. (Fig.2C). 2 C). After digestion with restriction enzymes, the PCR product is ligated into the pGEX vector and transformed into an E. coli host. Several of the transformants should be grown in mini-culture preparations to check for expression of the target protein. Glycerol stocks of the bacterial culture should be prepared for storage at −80 °C. Below is a map of pUC18 and the PCR product. (a) In order to clone just the lin-14 gene, which restriction enzyme(s) ... One is the lin-4 fragment ligated into the vector. ... You decide to generate a genomic DNA library of BamHI fragments cloned into a plasmid vector in E. coli.
Below is a diagram of the pcr product ligated into the plasmid.. substrate called X-gal and cleave the β-1,6 linkage to form a product that is bright blue. For each of the following sequences found on pBlue, list the step or steps (1-7 above) for which that sequence is ... you decide to use Polymerase Chain Reaction (PCR) to amplify the psy2 coding sequence based on the flanking ... PCR fragment into vector ... Part E Below is a diagram of the PCR product ligated into the plasmid. Drag the restriction enzyme names to the appropriate places on the plasmid diagram 19 kb 16 kb pCDNA3 p53 23 kb S 14 kb 10 kb Submit My Answers Give Up Part F E 1 kb 4 kb B 7 kb 9 kb help 16.4.2020 · The Ash1 coding sequence was amplified from a His-Ash1 plasmid reported previously (Martin et al., 2016a) and further assembled into a pEGFP-G3BP1 plasmid. For protein expression, G3BP1-coding DNA was cut by BamHI and EcoRI and ligated into the pGEX-2T plasmid in which the thrombin site had been replaced with a TEV site using the Q5 Site … In the diagram below, the chimeric plasmid carries resistance to ampicillin but the β-galactosidase gene has been disrupted. The bacteria are grown on a solid medium with ampicllin and X-gal. Cells resistant to ampicillin grow into colonies and white colonies (lac-) are those carrying the chimeric plasmid with the foreign DNA.
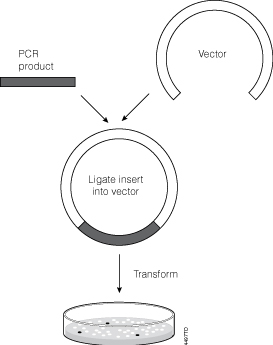
When the yeast gene is ligated into the plasmid, h erar2 s it w XbaI canlave(on n h eplasmi dan oncaf tr nof op ead ing f ramo t hyeasge ).T spoduc 2 bands, one is ~1250bp and the other is 5000bp. Colony 2's plasmid = VectorAlone (religated to itself) When there is no yeast gene ligated into the plasmid, Therefore, if a DNA fragment is ligated into the MCS, the lacZ gene will no longer function and will not produce the blue color. In the presence of X-gal, cells that contain a plasmid with a fragment inserted into the MCS will be white, whereas cells that contain a plasmid without a fragment inserted into the MCS will be blue. View Ligation and Transformation Problem Set.pdf from BMB 448 at Pennsylvania State University. Purification, Ligation, and Transformation Problem Set Read the Cloning & Sequencing Explorer Series The example below depicts the ligation of two sticky ends that were generated by EcoRI digestion: Usually, scientists select two different enzymes for adding an insert into a vector (one enzyme on the 5' end and a different enzyme on the 3' end). This ensures that the insert will be added in the correct orientation and prevents the vector from ...
Download scientific diagram | | Confirmation of recombinant pCAMBIA F+HN through PCR and restriction digestion. (A) Recombinant plasmid was digested with Nco1 and Bgl11 to confirm the presence of ... Below is a diagram of the PCR product ligated into the plasmid. Drag the restriction enzyme names to the appropriate places on the plasmid diagram. PCR amplification of cDNA synthesized with the Advantage RT-for-PCR Kit vs. cDNA synthesized with kits from two competitors. The indicated amounts of Human Lung Total RNA (Cat. # 636507) were used for reverse transcription, and 5% of each cDNA product was amplified by PCR using the Human G3PDH Control Amplimer Set (Cat. # 639005). To clone your gene of interest into pCR ® 2.1, you must first generate a PCR product. The PCR product is ligated into pCR ® 2.1 and transformed into competent cells. Since the PCR product can ligate into the vector in either orientation, individual recombinant plasmids need to be analyzed to confirm proper orientation.
ligase. enzyme that joins the cut gene and the cut plasmid. DNA ligation. a phosphodiester bond is created between the 3 prime hydroxyl of one nucleotide and the 5 prime phosphate of the next nucleotide. if ligation works the way we want it to. only our gene will ligate with the cut plasmid.
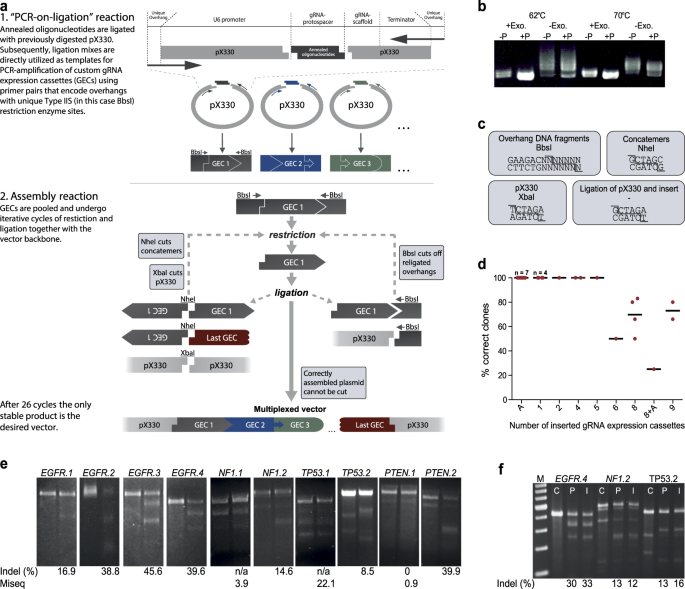
This diagram below provides an example of what the oligos may look like, in this example the oligos are to be ligated NcoI and XbaI restriction sites. ... If the PCR product is to be ligated into a de-phosphorylated vector, heat-inactivating the PNK enzyme may be a good idea to prevent it from phosphorylating the backbone vector and leading to ...
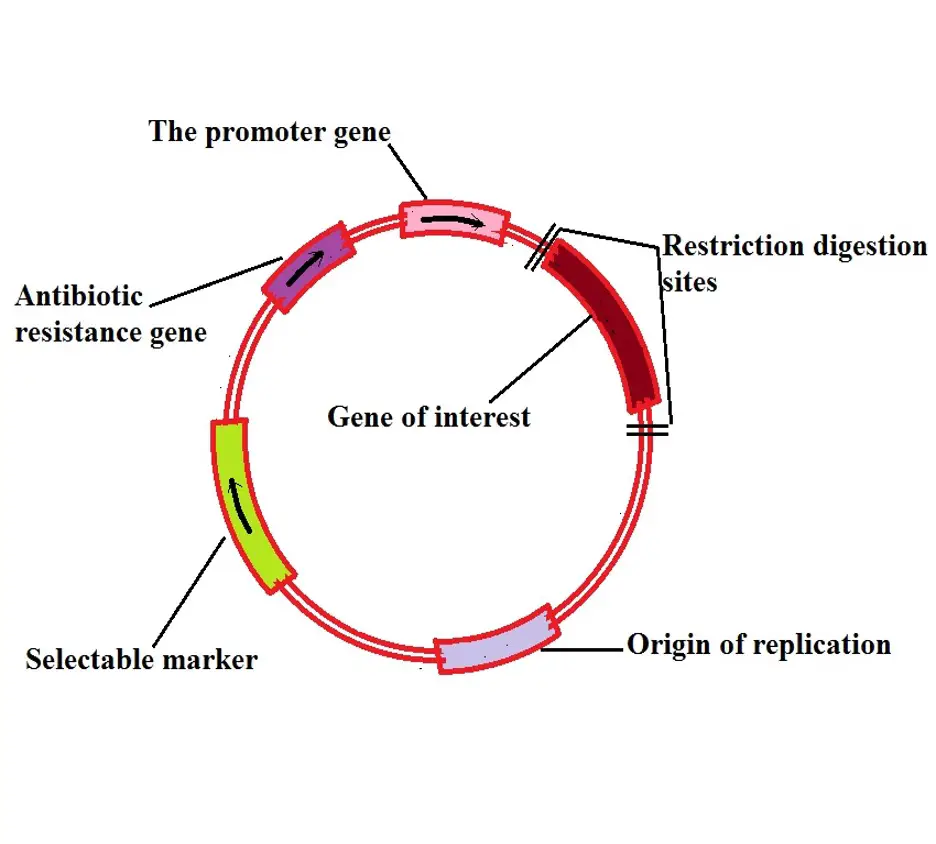
Once the gene of interest has been ligated enzymatically into the construct, this whole complex is ligated into bacterial plasmids (see Figure 3), ... The bacteria are then plated out. It is possible to tell from reporter genes (see below) ... Diagram of DNA sequence of a basic plasmid and incorporated construct.
Isolate your PCR product from the rest of the PCR reaction using a kit, such as the QIAquick PCR Purification Kit. The PCR product is now ready for restriction digestion. Digest your DNA: Set up restriction digests for your PCR product and recipient plasmid. Because you lose some DNA during the gel purification step, it is important to digest ...
PCR is an important technique in molecular biology and it was discovered by Kary Mullis in 1985 (Fig. 8). It is carried out in vitro and by it, upto billion copies of the target DNA sequence can be obtained from a single copy within few hours only. Outline of PCR: PCR is a technique which results in selective amplification of a selected DNA ...
The diagram below shows a section of human DNA that contains an imaginary gene for video game proficiency (the vgp gene), shown in red. ... Below is a diagram of the PCR product ligated into the plasmid. Drag the restriction enzyme names to the appropriate places on the plasmid diagram. 16Kb- B 19Kb- X 4Kb- E 9Kb- H
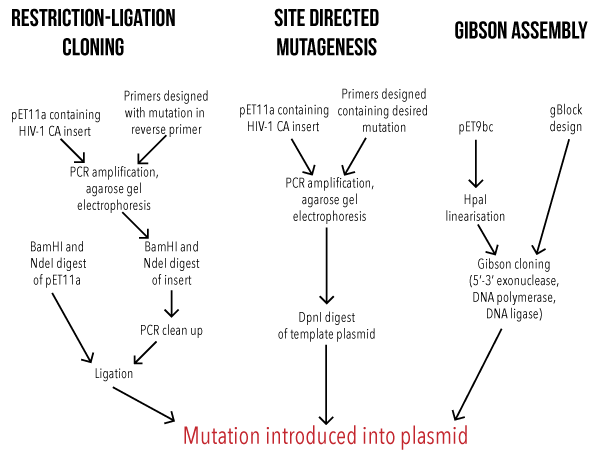
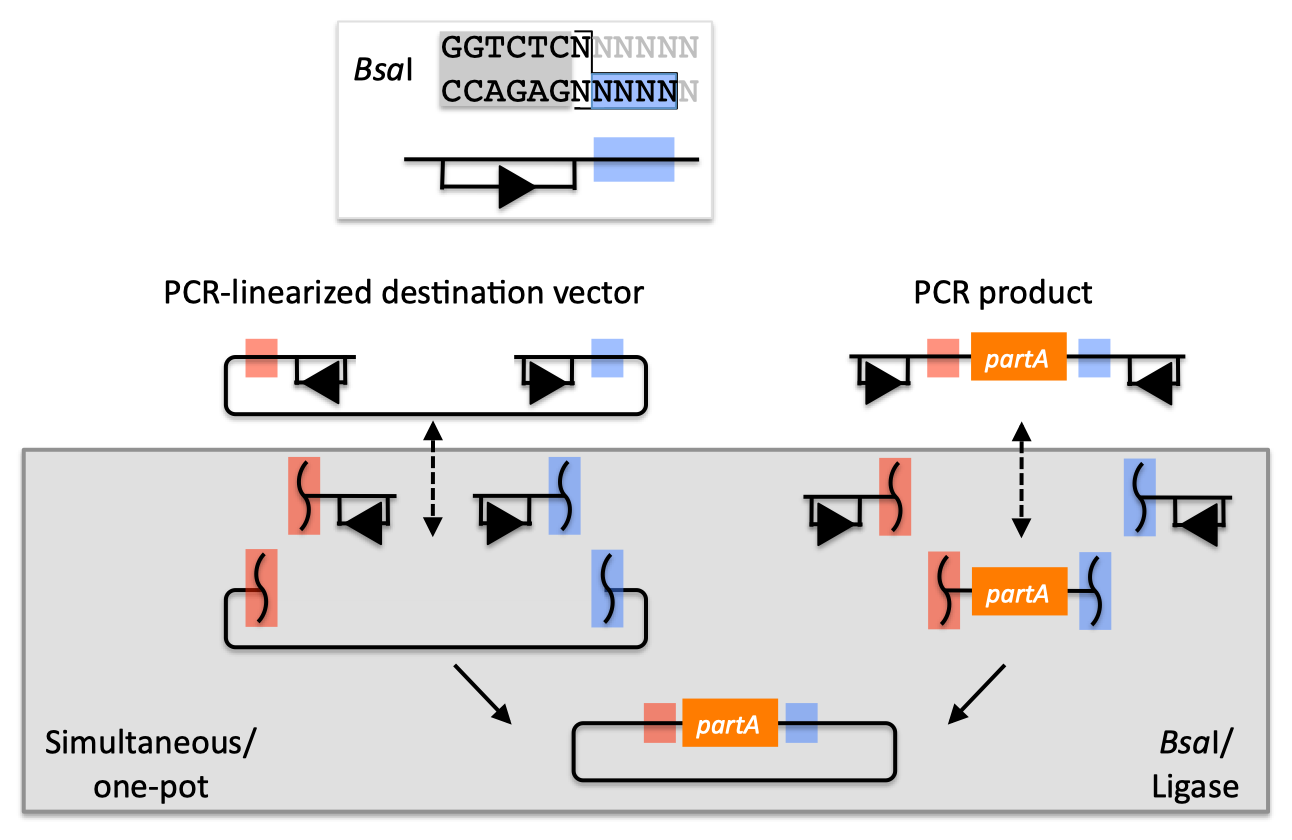
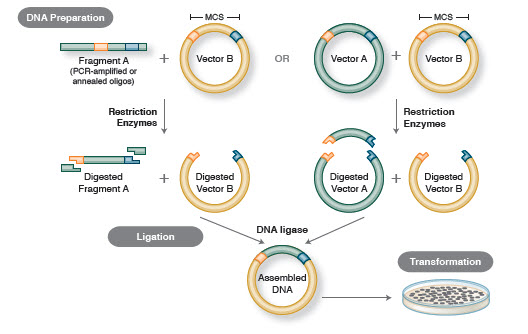
PCR cloning differs from traditional cloning in that the DNA fragment of interest, and even the vector, can be amplified by the Polymerase Chain Reaction (PCR) and ligated together, without the use of restriction enzymes. PCR cloning is a rapid method for cloning genes, and is often used for projects that require higher throughput than traditional cloning methods can accommodate.
A vector that ligates upon itself will get transformed more efficiently into E. coli and will thus decrease the efficiency of the cloning reaction. 3. Tips for a successful restriction digest: a. If your insert is a PCR product, you will probably add the restriction sites to the 5' end of both PCR primers.
The diagram below represents a section of the human genome. The coding sequence of a gene, YFG, is shown ... You would like to use PCR to amplify (make many copies of) the boxed section of the DNA sequence below: ... when you insert this DNA into a plasmid and transform it into the bacteria, you get no hormone production. Give two valid reasons ...
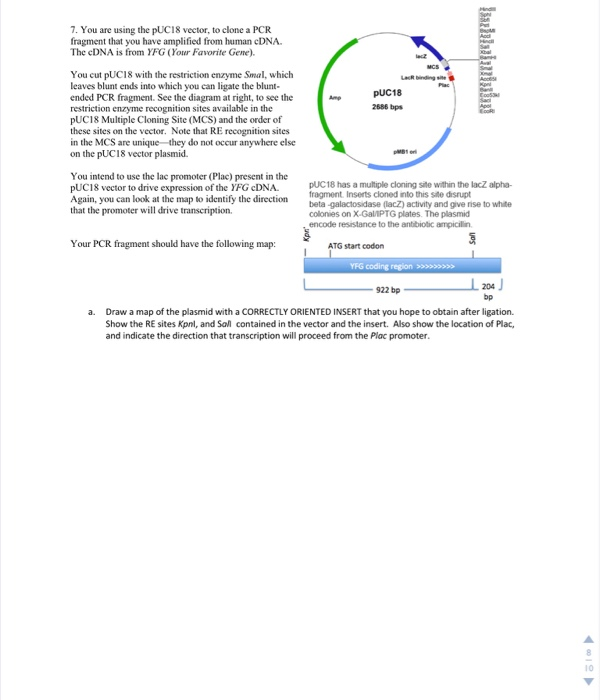
Q9: Shown below is a diagram for a plasmid vector you want to use to clone a gene. The diagram shows the location of the recognition sites for four restrictions enzymes, BamHI (B), EcoRI (E), HindIII (H), and XhoI (X). The genes encoding beta-lactamase (AmpR) and beta-galactosidase (lacZ) are indicated.
5.8.2009 · The J-lat A1 cellular model. (A) Diagram of the HIV-1 construct integrated in position ChXp21.1 of J-lat A1.The 5′-LTR is followed by the packaging signal, the major splice donor site (SD) and a portion of the gag gene fused to the Rev-responsive element (RRE). The Tat acceptor site SA7 precedes a cassette containing the Tat101 gene fused to a flag tag (f-Tat101), an …
Below is the map of the plasmid . Promoter Sal1 Xho1 Kpn1 . a) Your task is to design a strategy to insert the yeast gene into the bacterial plasmid. With which one set of enzymes would you choose to cut the yeast genomic DNA and the plasmid, out of the following choices? Explain . why you selected that pair.
A piece of DNA is ligated with the plasmid pBR322 which has been digested with the restriction enzyme PstI. Following transformation of the ligation products into E.coli, the cells are grown on plates containing ampicillin, tetracycline or ampicillin & tetracycline.
2.6.2005 · To construct the plasmid for disrupting the CAN1 locus with SPT15-Rluc, the 800-bp SPT15 promoter region was amplified from S. cerevisiae FY250 genomic DNA using primers oDM0298 and oDM0274, which incorporated unique 5′ and 3′ BamHI and HindIII sites, respectively, into the PCR product.
Below is a diagram of the PCR product ligated into the plasmid. Drag the restriction enzyme names to the appropriate places on the plasmid diagram. Question: Below is a diagram of the PCR product ligated into the plasmid. Drag the restriction enzyme names to the appropriate places on the plasmid diagram.
The PCR products (4774 bp, containing homologous sequences) were verified by electrophoresis on 0.9% agarose gel. The remaining 17 μL PCR products were mixed with 0.5 μL DpnI enzyme (BioLabs, Ipswich, USA), centrifuged, and incubated at 37°C for 3 hours. Transformation of the pEGFP-N1-HA plasmid into E. coli
Q.9. How is Ti plasmid of Agrobacterium tumefaciens modified to convert it into a cloning vector? A.9. The Ti plasmid is a tumour-inducing plasmid. The genes responsible for its pathogenic nature are either removed or altered so that it does not harm the plants and only delivers the gene of interest. Q.10.
Below is a map of pUC18 and the PCR product. (a) In order to clone just the lin-14 gene, which restriction enzyme(s) ... One is the lin-4 fragment ligated into the vector. ... You decide to generate a genomic DNA library of BamHI fragments cloned into a plasmid vector in E. coli.
After digestion with restriction enzymes, the PCR product is ligated into the pGEX vector and transformed into an E. coli host. Several of the transformants should be grown in mini-culture preparations to check for expression of the target protein. Glycerol stocks of the bacterial culture should be prepared for storage at −80 °C.
The PCR products obtained for P. atlantica with each primer set were ligated into the plasmid pCR2.1 and sequenced (see Methods and Materials). The primary products for both primer sets had the IS 492 right- and left-end sequences separated by a 5-bp junction sequence (Fig. (Fig.2C). 2 C).





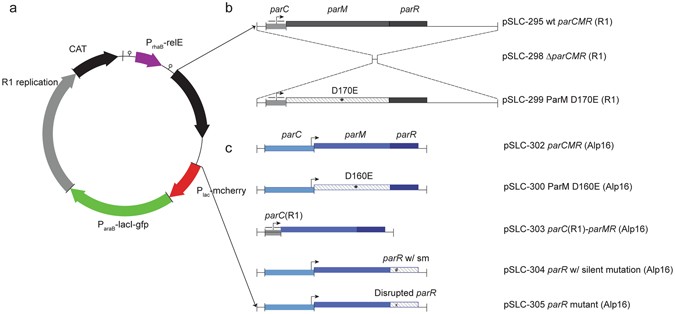

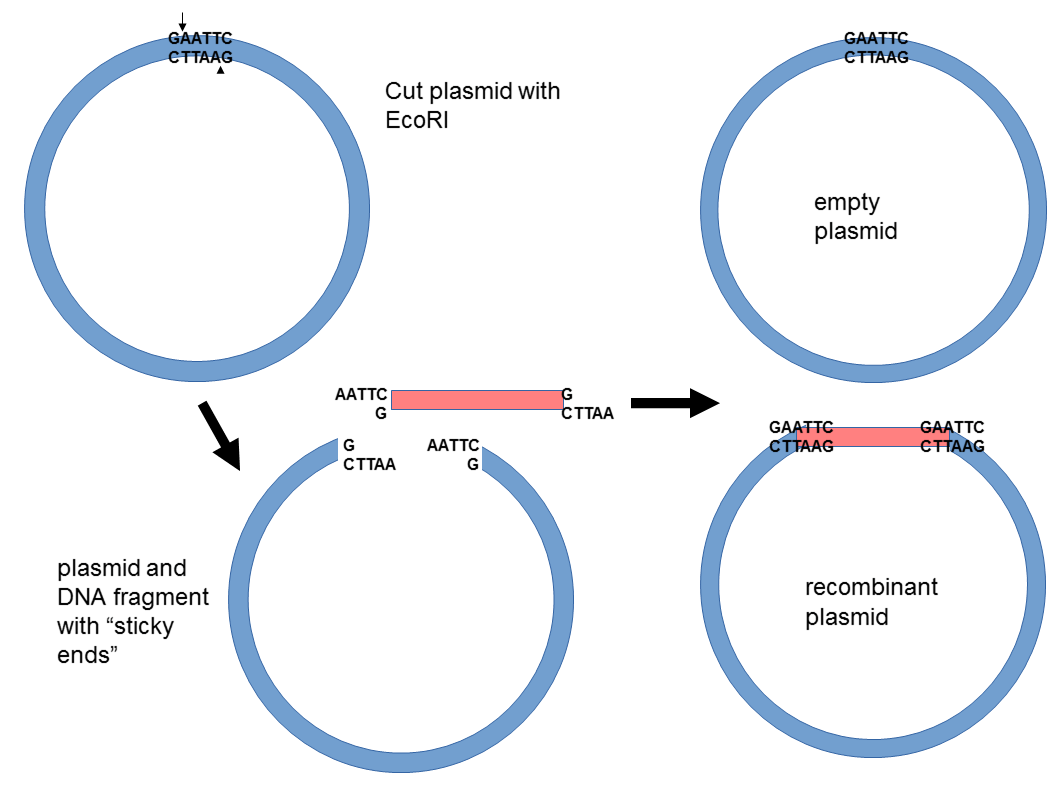





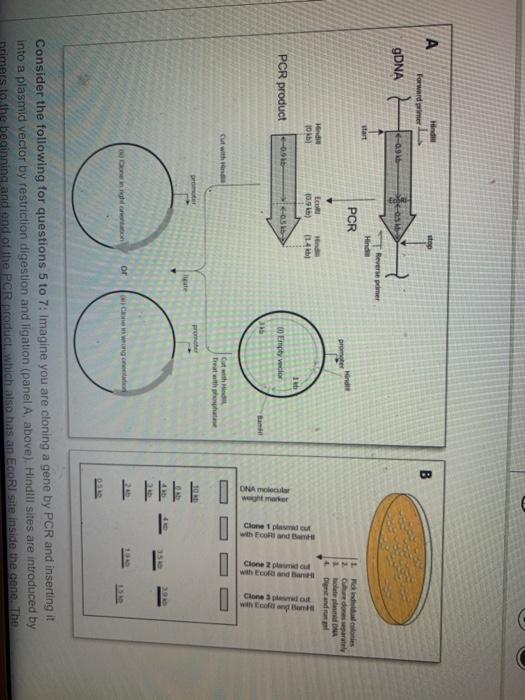





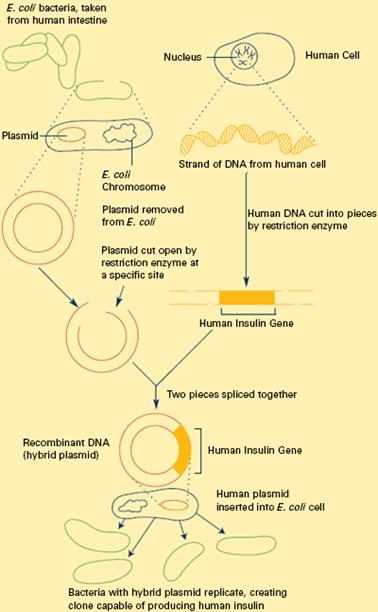

0 Response to "42 below is a diagram of the pcr product ligated into the plasmid."
Post a Comment