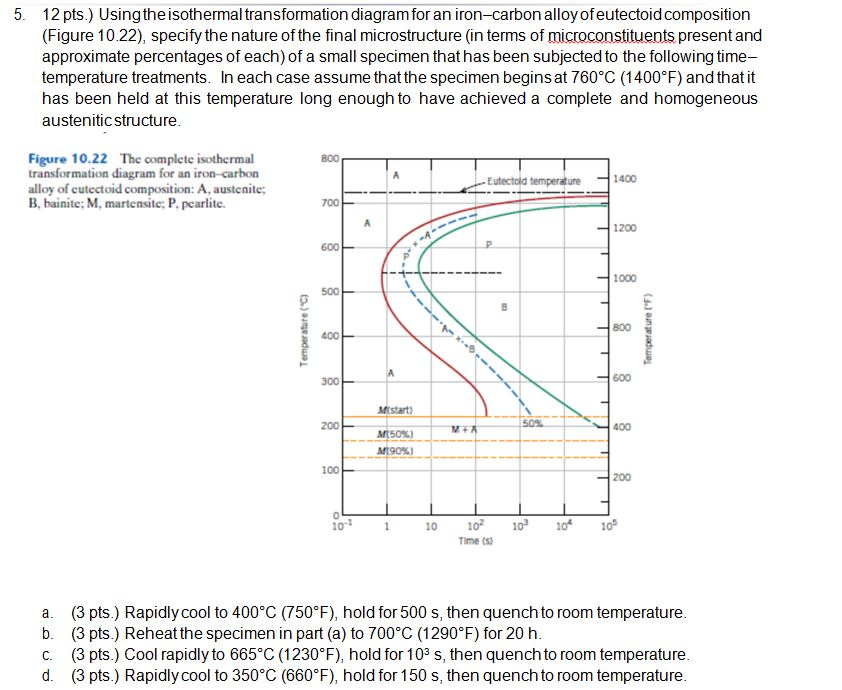
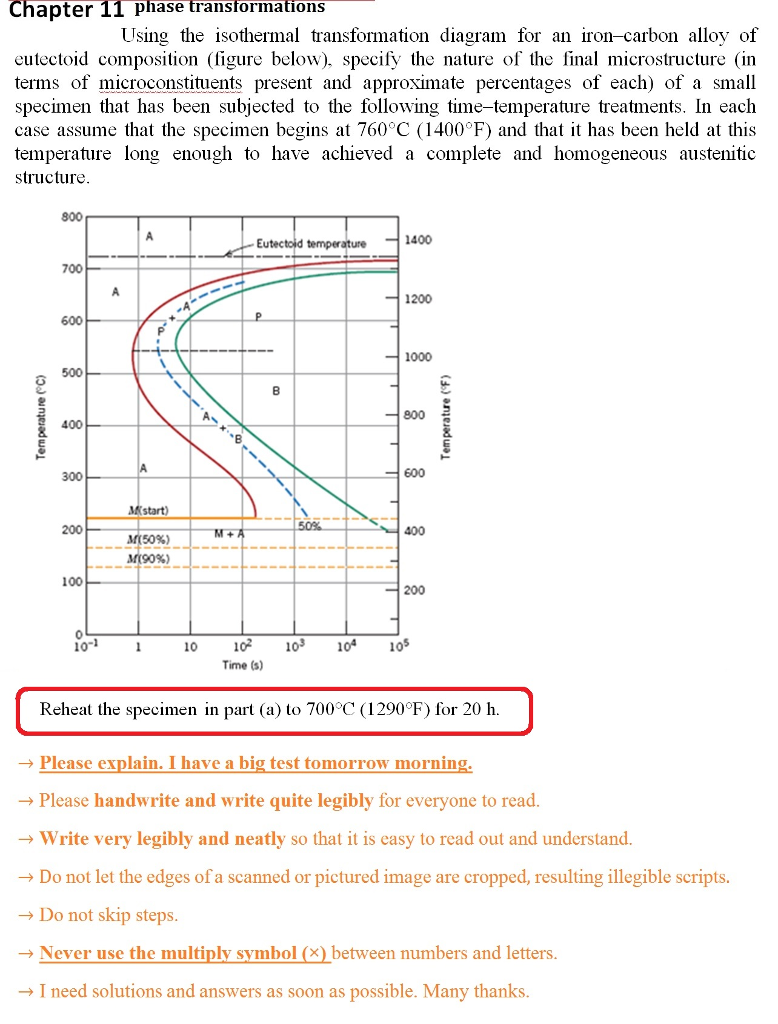
44 using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition
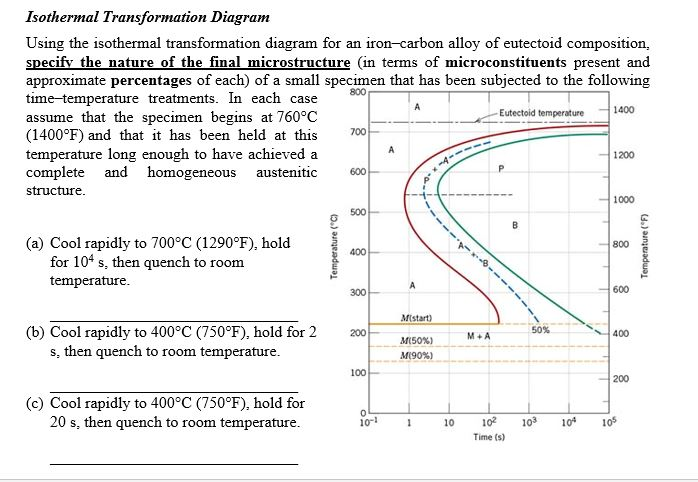
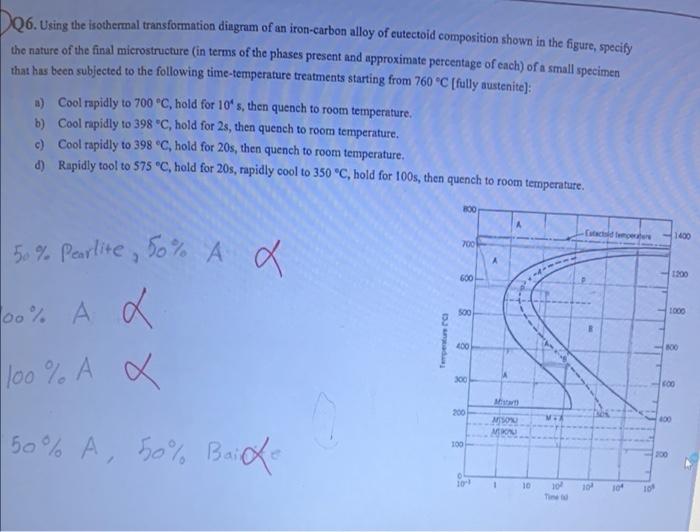
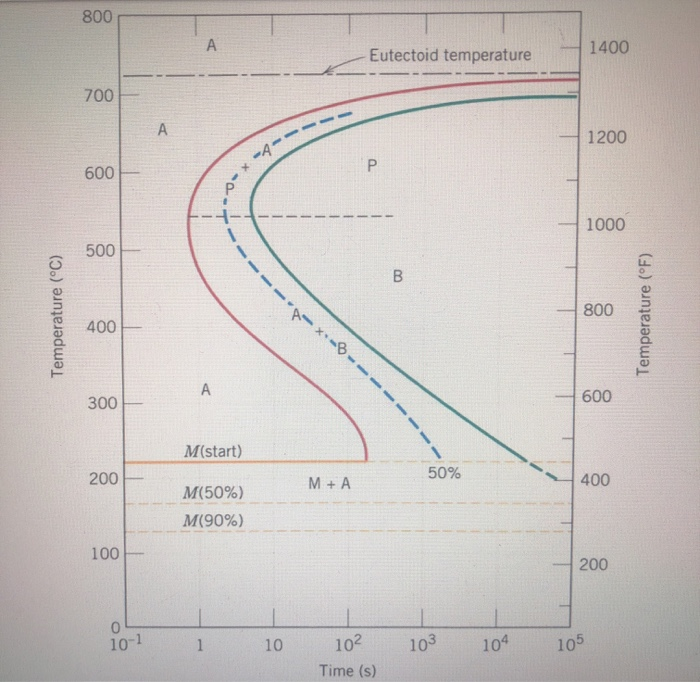
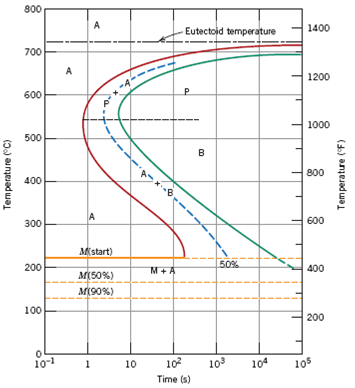
Question: Using the isothermal transformation diagram for an ironâ€"carbon alloy of eutectoid composition (Figure 10.22), specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following timeâ€"temperature treatments. In each case assume that the specimen begins at ... Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition (see Figure 10.22 below), specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following temperature treatments.
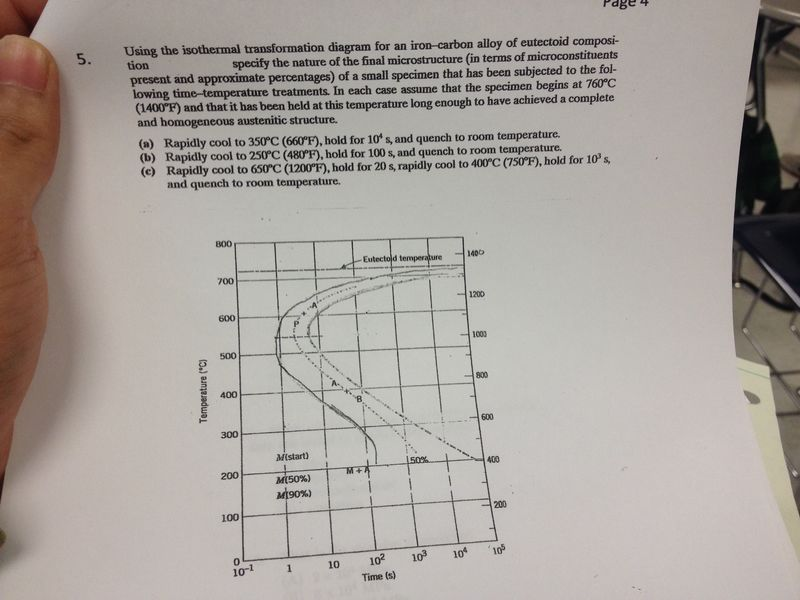
Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition, specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following time-temperature treatments.
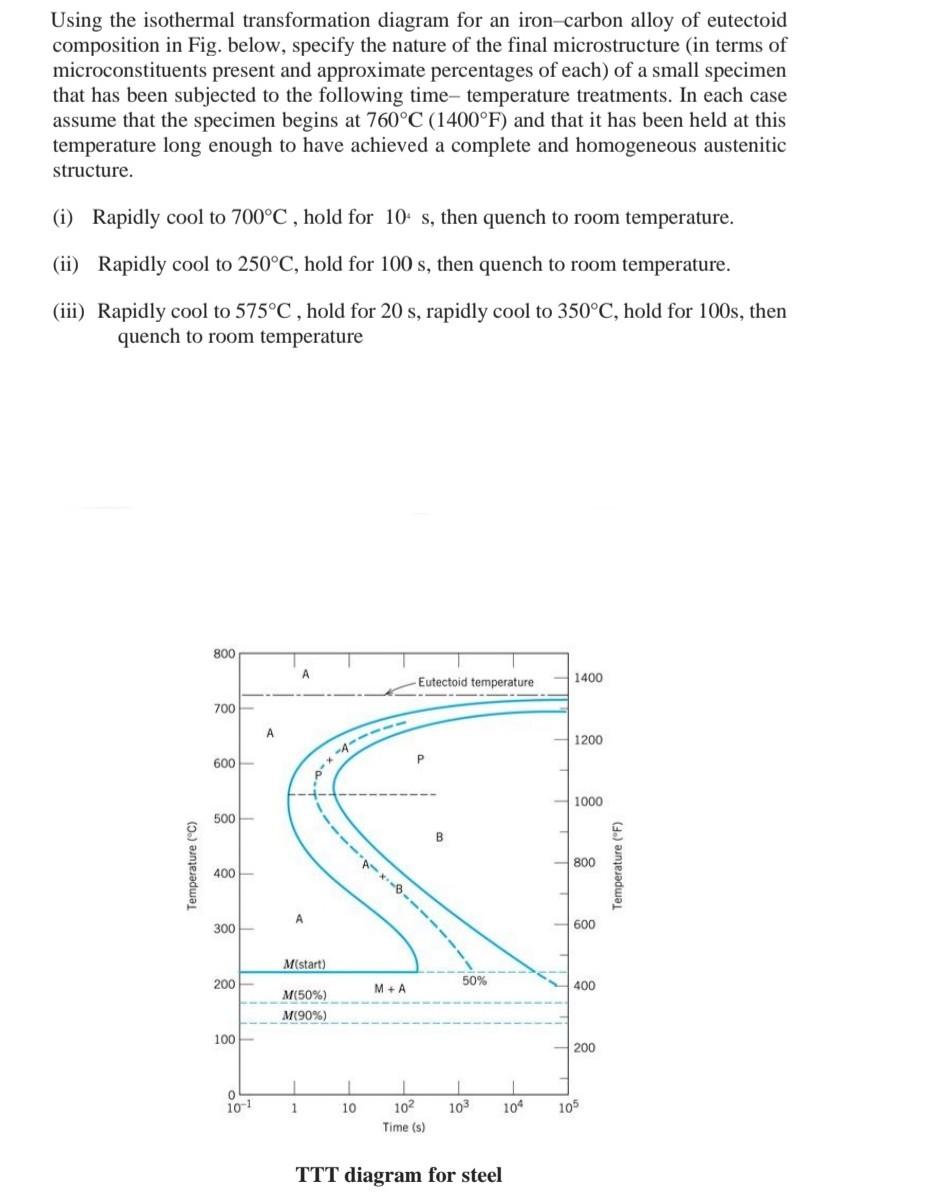
Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition
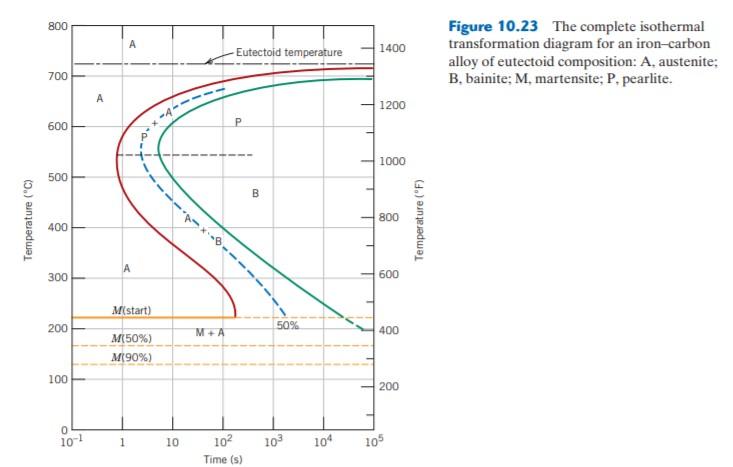
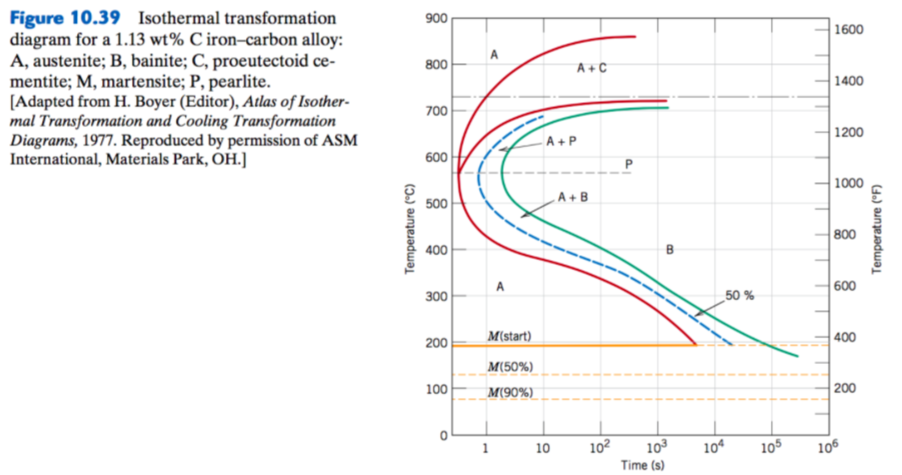
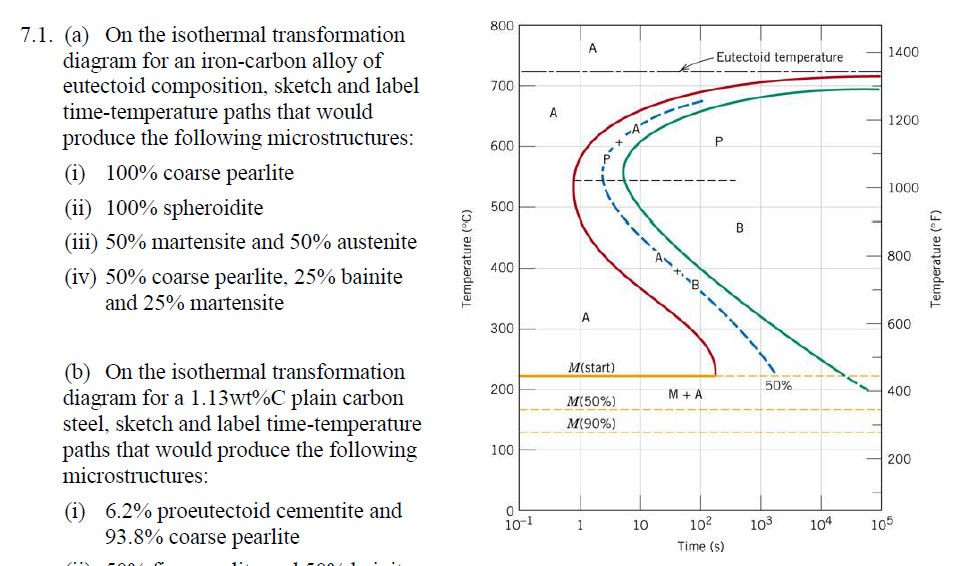
Using the isothermal transformation diagram for a 1.13wt%C steel alloy determine the final microstructure (in terms of just the microconstituents present) of a small specimen that has been subjected to the following time-temperature treatments. In each case assume that the specimen begins at 920oC, and that Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition (Figure 10.23), specify the nature of the final microstructure (in terms of microconstituents presentand approximate percentages of each) of a small specimen that has been subjected to the following time -temperature treatments. Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition (Figure 1), specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following time-temperature treatments.
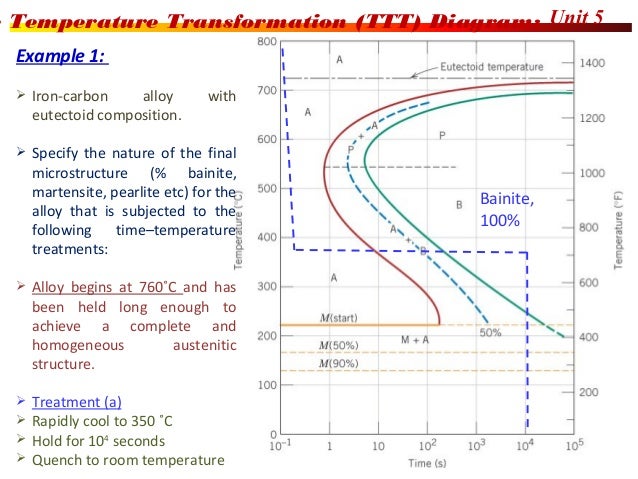
Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition. Unlike the isothermal decomposition of phase constituents by diffusion, martensite is not a phase associated with thermal equilibrium. Thus, it does not appear on the iron-carbon equilibrium phase diagram. It may be thought of as a transformation product that is … Using the Animated Figure 10.22, the isothermal transformation diagram for an alloy of eutectoid composition, specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following temperature treatments. Eutectoid steel is the stage in the iron carbide diagram, in which the steel contains complete pearlite in its microstructure. Hypo eutectoid steels contain less than 0.83% carbon where ferrite is in combination with cementite having thin lamellars called coarse pearlite. In which percentage of ferrite is decreasing. ttt diagram is a plot of temperature versus the logarithm of time for a steel alloy of the complete isothermal transformation diagram for an iron-carbon alloy.c metastable equilibrium diagram and ttt diagrams for plain carbon hypoeutectoid, eutectoid and hypereutectoid steels m s (a) fe-fe 3 c metastable phase diagram (b) ttt diagram for …
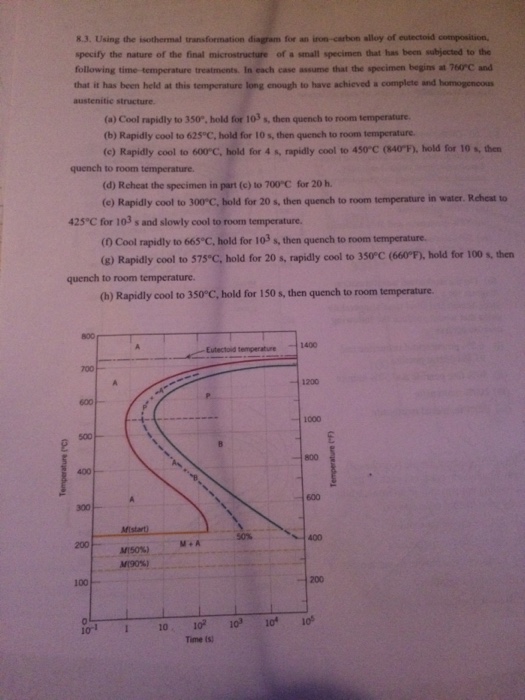
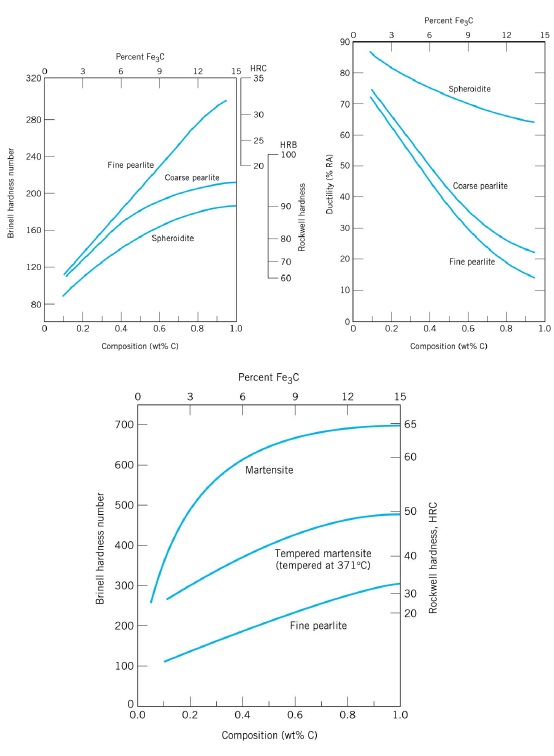
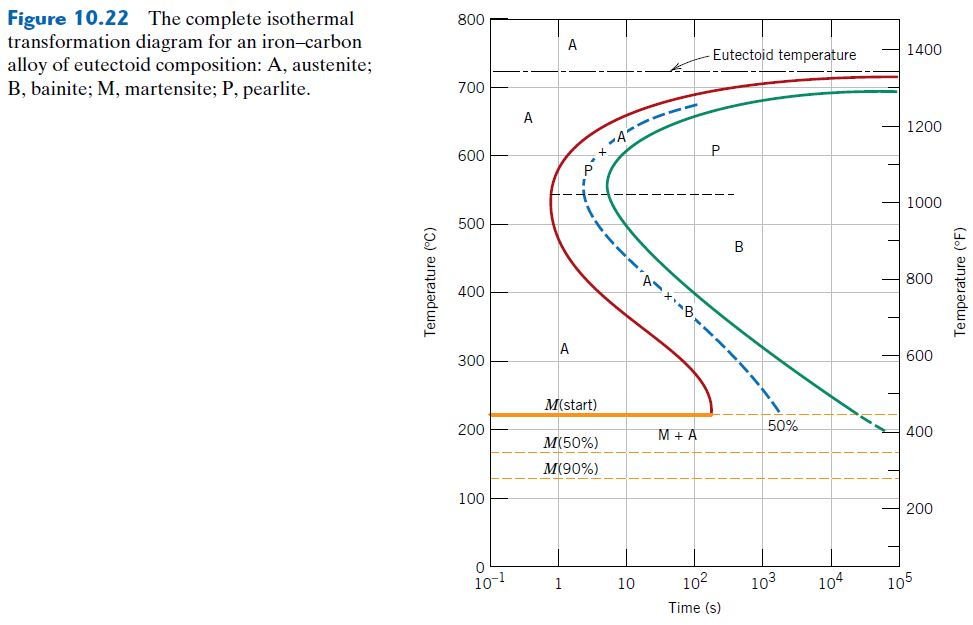
For heat treatment of steels, the first resource to become familiar with is the iron–cementite equilibrium phase diagram, which shows the equilibrium phases in iron–carbon alloys for a given temperature and composition.The iron–carbon equilibrium phase diagram (10) presented in Figure 1 shows carbon levels up to 7 wt.%, but steels are iron–carbon alloys only up to … Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition, specify the nature of the final microstructure of a small specimen that has been subjected to the following time-temperature treatments. 10.14 This problem asks us to determine the nature of the final microstructure of an iron-carbon alloy of eutectoid composition, that has been subjected to various isothermal heat treatments. Figure 10.14 is used in these determinations. (a) 50% coarse pearlite and 50% martensite (b) 100% spheroidite 10.18 Using the isothermal transformation diagram for an iron–carbon alloy of eutectoid composition. (Figure 10.22), specify the nature of the final ...12 pages
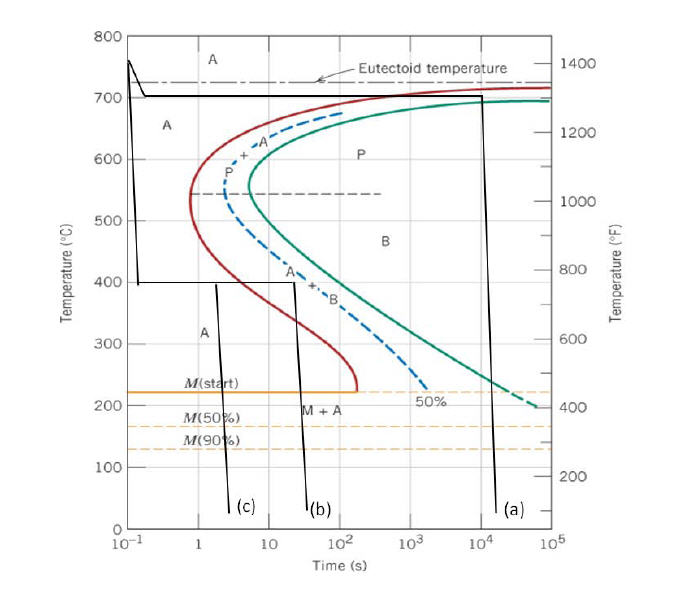
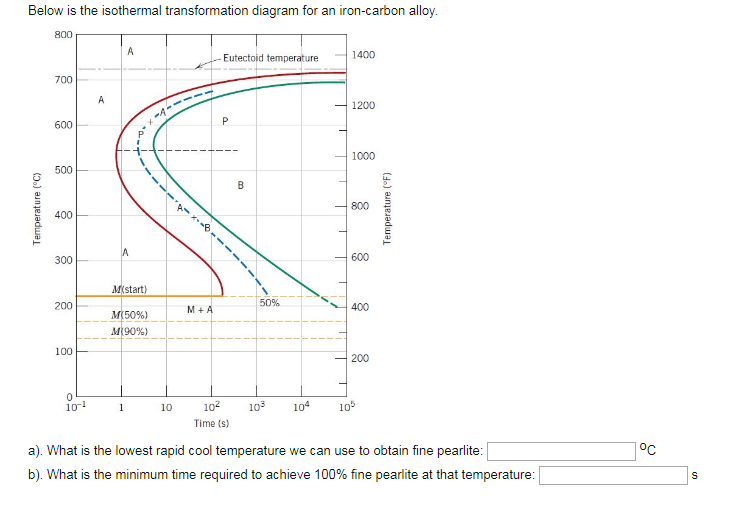
10.19 Using the isothermal transformation diagram for an iron - carbon alloy of eutectoid composition (Figure 10.22), specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following time - temperature treatments. In each case assume that the specimen begins at 760°C ... Answer to: Use the isothermal transformation diagram for an iron?carbon alloy of eutectoid composition below (Figure 10.11). For each...1 answer · Top answer: a) The austenite is rapidly cooled to 700 C and held for at a constant temperature for 104 seconds, and further cooling to room temperature... Explanation: In peritectoid reactions, two solid phases isothermally and reversibly transform into a solid with a third and different composition. It is also known as an upside-down eutectoid reaction. Such reactions are commonly found in Ni-Zn, Fe-Nb, Cu-Sn, and other systems. using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition (figure below), specify the nature of the final microstructure (in terms of micro-constituents present and approximate percentages of each) of a small specimen that has been subjected to the following time-temperature treatment: rapidly cool to 625oc, …
• Eutectoid composition, C0= 0.76 wt% C • Begin at T > 727°C • Rapidly cool to 625 oC • Hold T (625 oC) constant (isothermal treatment) Fig. 12.14, Callister & Rethwisch 9e. [Adapted from H. Boyer (Editor), Atlas of Isothermal Transformation and Cooling Transformation Diagrams, 1977. Reproduced by permission of ASM
EXAMPLE PROBLEM 11.1 Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition (Figure 11.14), specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages) of a small specimen that has been subjected to the following time-temperature treatments.
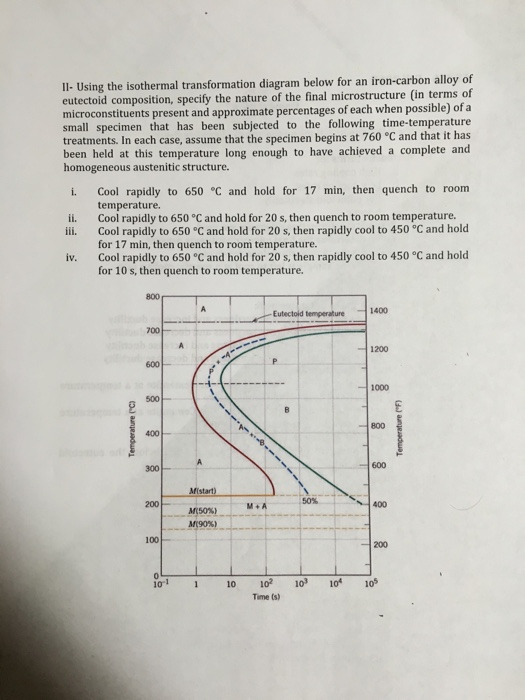
Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition (Figure), specify the nature of the final microstructure (in terms of micro constituents present and approximate percentages of each) of a small specimen that has been subjected to the following time-temperature treatments.
Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition, specify the nature of the final microstructure (in terms of micro-constituents present and approximate percentages of each) of a small specimen that has been subjected to the following time temperature treatments.
The Fe-C phase diagram depicted in Chapter 11 illustrates an important characteristic in the steel composition range which is called the ‘eutectoid’ which refers to the composition of a solid phase which, upon cooling, undergoes a univariant transformation into two, or more, other solid phases. 4 For a carbon steel, the eutectoid point ...
Austenite, also known as gamma-phase iron (γ-Fe), is a metallic, non-magnetic allotrope of iron or a solid solution of iron, with an alloying element. In plain-carbon steel, austenite exists above the critical eutectoid temperature of 1000 K (727 °C); other alloys of steel have different eutectoid temperatures. The austenite allotrope is named after Sir William Chandler Roberts …
Using the supplied isothermal transformation diagram for an iron-carbon alloy of eutectoid composition, specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following time-temperature treatments.
10.18 Using the isothermal transformation diagram for an iron–carbon alloy of eutectoid composition (Figure 10.22), specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following time–temperature treatments. In
Problem 2 Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition (Figure 10.22), specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following time- temperature treatments.
Using the Animated Figure 10.22, the isothermal transformation diagram for an alloy of eutectoid composition, specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following temperature treatments.
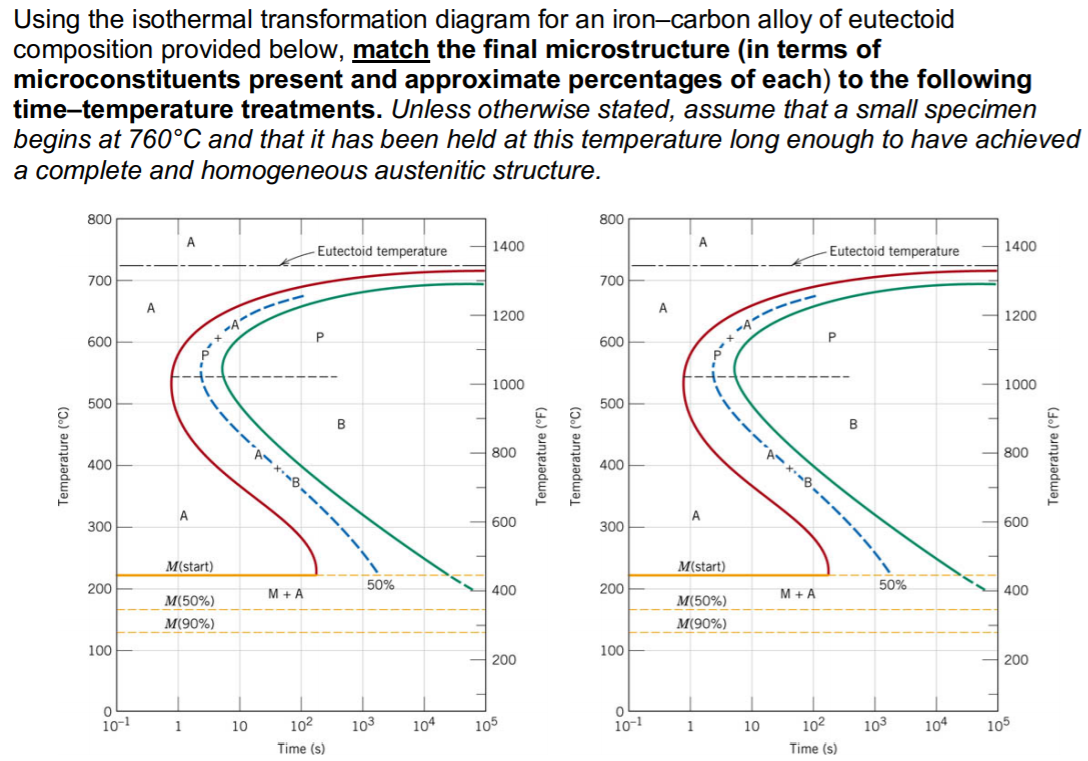
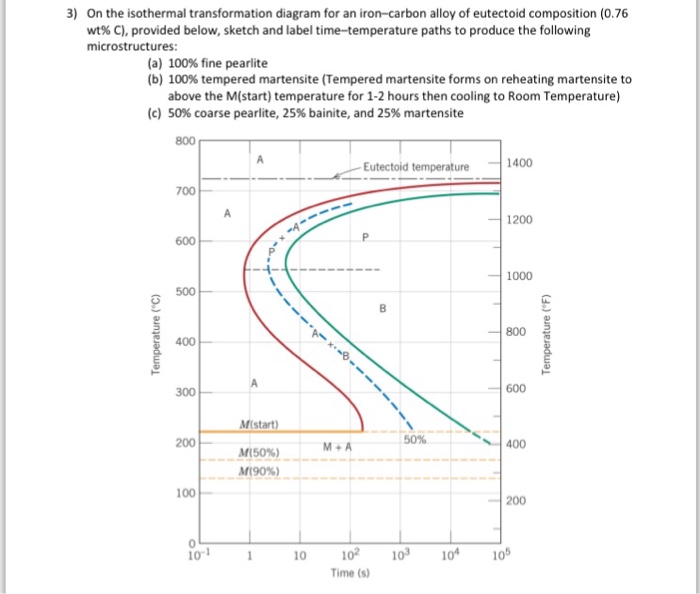
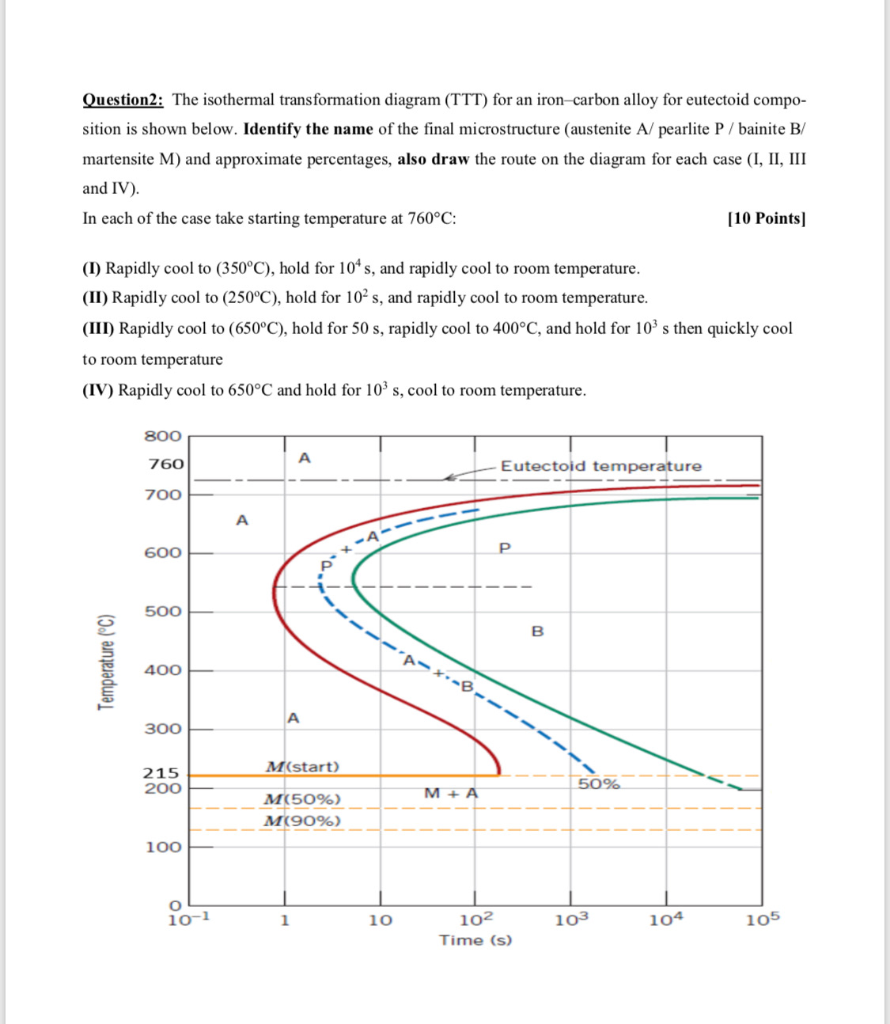
Solution for Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition, sketch (mark the microconstituents, eg. a-Fe, ...
Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition, specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following time-temperature treatments.
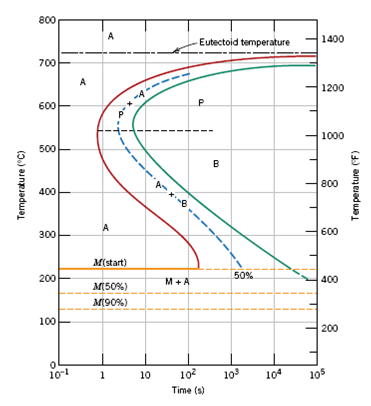
Time-Temperature-Transformation (TTT) diagram or S-curve refers to only one steel of a particular composition at a time, which applies to all carbon steels.This diagram is also called as C-curve isothermal (decomposition of austenite) diagram and Bain’s curve.The effect of time-temperature on the microstructure changes of steel can be shown by the TTT diagram.
10.18 Using the isothermal transformation diagram for an iron–carbon alloy of eutectoid composition. (Figure 10.22), specify the nature of the final ...27 pages
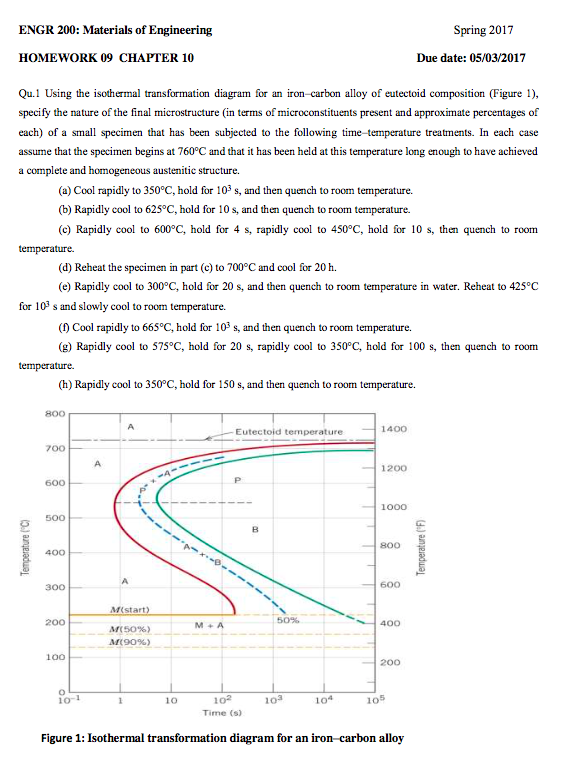
Using the isothermal transformation diagram for an ironcarbon alloy of eutectoid composition figure 1 specify the nature of the final microstructure in terms of microconstituents present and approximate percentages of each of a small specimen that has been subjected to the following timetemperature treatments.
Using the isothermal transformation diagram for an ironcarbon alloy of eutectoid composition figure 1022 specify the nature of the final microstructure in terms of microconstituents present and approximate percentages of each of a small specimen that has been subjected to the following timetemperature treatments.
Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition (Figure), specify the nature of the final microstructure (in terms of micro constituents present and approximate percentages of each) of a small specimen that has been subjected to the following time-temperature treatments.
Using the isothermal Transformation Diagram for An Iron-carbon Alloy Of Eutectoid Composition. solved 10 19 using the isothermal transformation diagram 10 19 using the isothermal transformation diagram for an iron carbon alloy of eutectoid position figure 10 22 specify the nature of the final microstructure in terms of microconstituents present and approximate percentages of each of a small ...
Fig.4: Time temperature transformation (schematic) diagram for plain carbon eutectoid steel t 1 t 2 t 3 t 4 t 5 M F, Martensite finish temperature M 50, 50% Martensite M S, Martensite start temperature Metastable austenite +martensite Martensite e 0 100 e Log time Hardness A e1 T 2 T 1 50% T T 2 1 Pearlite Fine pearlite Upper bainite Lower bainite
Why adding just a small amount of carbon to iron results in an alloy that is so much stronger than the base metal? In this course, you will learn how a ...Mar 8, 2016
Solution we are called upon to consider the isothermal transformation of an iron carbon alloy of eutectoid composition. 100 79 ratings using the isothermal transformation diagram for an ironcarbon alloy of eutectoid composition figure 1022 specify the nature of the final microstructure in terms of microconstituents present and approximate ...
Composition greater than that of the eutectoid. Hypoeutectoid. Composition less than that of the eutectoid. Martensite. The metastable iron-carbon solid solution phase with an acicular, or needle like, microstructure produced by a diffusionless transformation associated with the quenching of austenite. Normalizing. A simple heat treatment ...
Eutectoid. When the solution above the transformation point is solid, rather than liquid, an analogous eutectoid transformation can occur. For instance, in the iron-carbon system, the austenite phase can undergo a eutectoid transformation to produce ferrite and cementite, often in lamellar structures such as pearlite and bainite.This eutectoid point occurs at 723 °C (1,333 …
the iron-carbon alloy system. A sample of the eutectoid composition is cooled from a single-phase region (γ) to a temperature (T) below the eutectoid temperature (T E). The following diagram shows a part of the iron-carbon phase diagram. Concentrations C 1, C 2, C 3, C 4 are various equilibrium ( stable as well as metastable ) concentrations ...
Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition (Figure 1), specify the nature of the final microstructure (in terms of microconstituents present and approximate percentages of each) of a small specimen that has been subjected to the following time-temperature treatments.
Using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition (Figure 10.23), specify the nature of the final microstructure (in terms of microconstituents presentand approximate percentages of each) of a small specimen that has been subjected to the following time -temperature treatments.
Using the isothermal transformation diagram for a 1.13wt%C steel alloy determine the final microstructure (in terms of just the microconstituents present) of a small specimen that has been subjected to the following time-temperature treatments. In each case assume that the specimen begins at 920oC, and that















0 Response to "44 using the isothermal transformation diagram for an iron-carbon alloy of eutectoid composition"
Post a Comment