40 water molecular orbital diagram
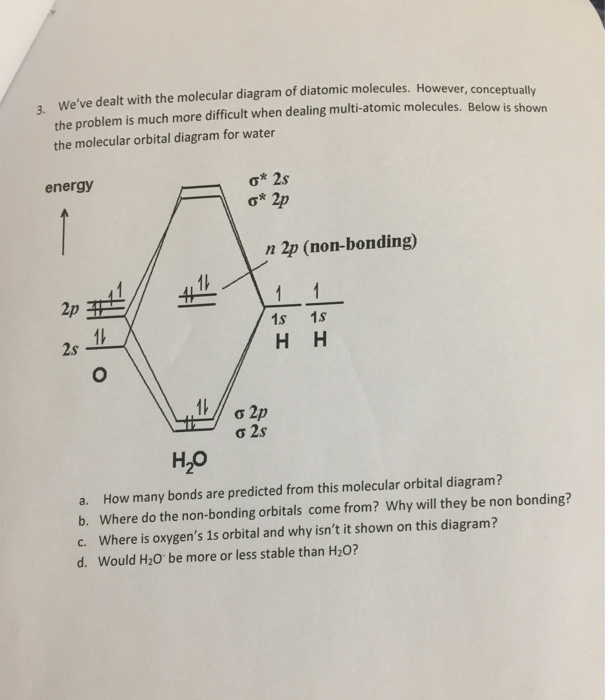
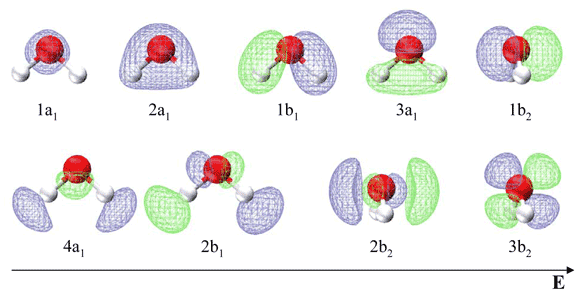
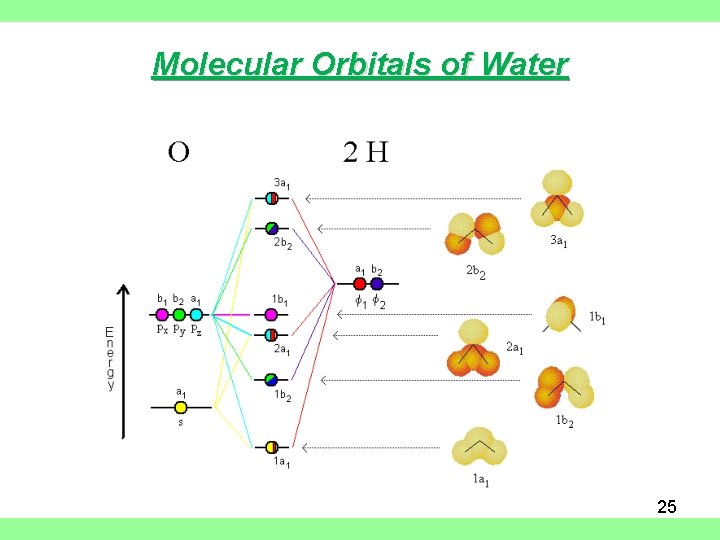
Water ( H 2 O) is an isolated molecule having five occupied and three unocc …. View the full answer. Transcribed image text: Report Sheet: Molecular Orbitals of Water 1. Match the orbital designations (1a, 2a, 3a, 1b, 1b, or 2b,) with each of the following possible combinations of atomic orbitals: a. 0+H +H. Orbital Designation b. 0:p+ H+H. Molecular orbital energy level diagram for H2O.Moving to a triatomic molecule, such as water, can complicate things further, given that an energy diagram would now need to be "three-sided" since three atoms are now involved. In the case of water, the simpler "two-sided" diagram can still be used, if we treat the two H 1s orbitals together.
molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104 ...

Water molecular orbital diagram
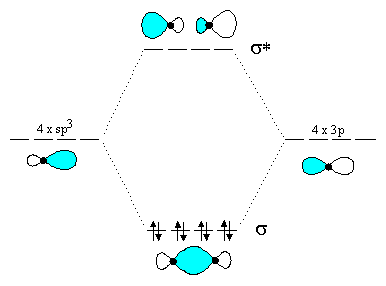
Here, using density functional theory and post-Hartree-Fock methods, we reveal two hydrogen bonding molecular orbitals crossing the hydrogen-bond's O and H atoms in the water dimer. Energy ... For two equivalent bonds (as the O-H bonds here are expected to be) with a bond angle θ, the hybridization of the localized bond orbital is given by s p λ where. λ = − 1 cos. . θ. Applying this to the known bond angle of water, we find that the O-H bond orbitals are ≈ s p 4, that is, 80% p and 20% s. So they are slightly more p in ... Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
Water molecular orbital diagram. Sep 04, 2021 · The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. The net contribution of the electrons to the bond strength of a molecule is identified by determining the bond order that results from the filling of the molecular orbitals by electrons. Create an initial 3D structure¶. Using Avogadro, we can create an initial structure of the water molecule. Simply choose the Draw Tool (), change the Element to Oxygen, tick the box for Adjust Hydrogens, and click anywhere in the View Window.This will draw a single oxygen atom, and hydrogens will be added automatically to satisfy the oxygen atom's valency, giving H2O. Molecular Orbitals of Water. Using the MNDO approach (Modfied Neglect of Diatomic Overlap), the shape of the water molecule has been optimized and, among other characteristics, we obtaine the molecule's orbitals. This approach just deals with valence electrons, in consequence, we have eight electrons in four occupied molecular orbitals. Simple Molecular Orbital (MO) diagram of H 2O Simple MO of H 2 O In contrast to localizing electrons within their atomic orbitals in valence bond theory, the molecular orbital approach considers electrons to be delocalized across the entire molecule. The simple MO diagram of H 2O is shown on the right.
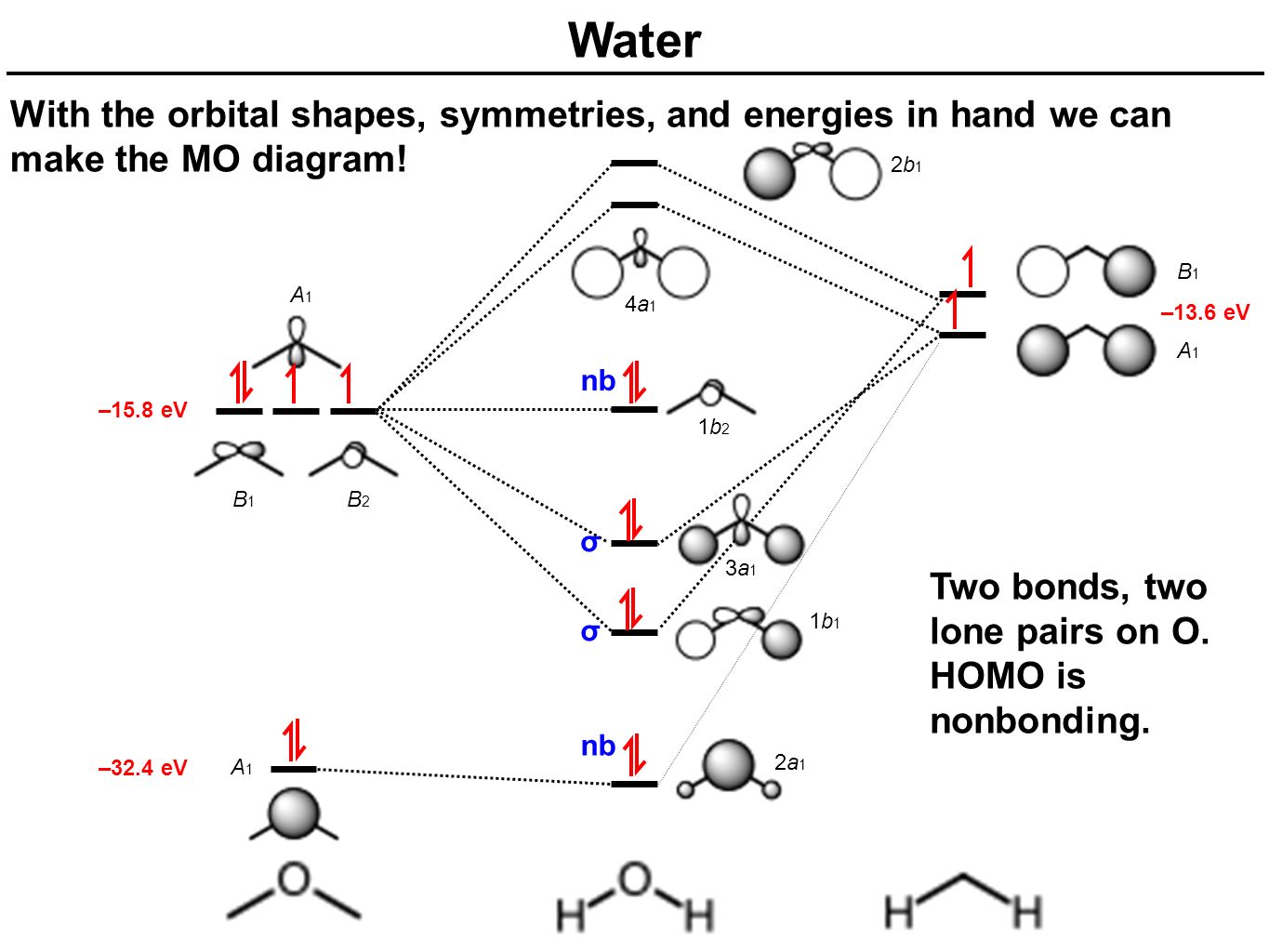
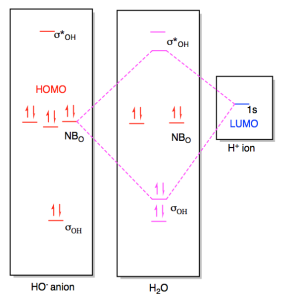
Molecular Orbital diagram of water (H2O) The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound. Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure. The molecular orbitals in the water molecule are classified as a 1, b 1 or b 2 orbitals, as determined by their symmetry properties. This labelling of the orbitals is analogous to the use of the s-p and g-u classification in linear molecules. In addition to the symmetry properties of the atomic orbitals we must consider their relative energies ... The molecular orbital theory explanation (Bent's rule) is that lowering the energy of the oxygen atom's nonbonding hybrid orbitals (by assigning them more s character and less p character) and correspondingly raising the energy of the oxygen atom's hybrid orbitals bonded to the hydrogen atoms (by assigning them more p character and less s ... The atomic orbitals combine to produce the following molecular orbital diagram: Here the 2 p g orbital is occupied by two electrons to give a total bond order of three. This corresponds well with the Lewis structure ( ), although the orbital approach tells us that there is one s and two p .
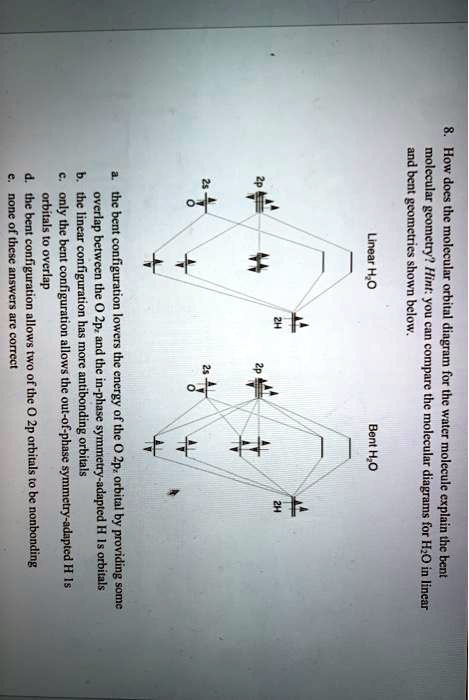
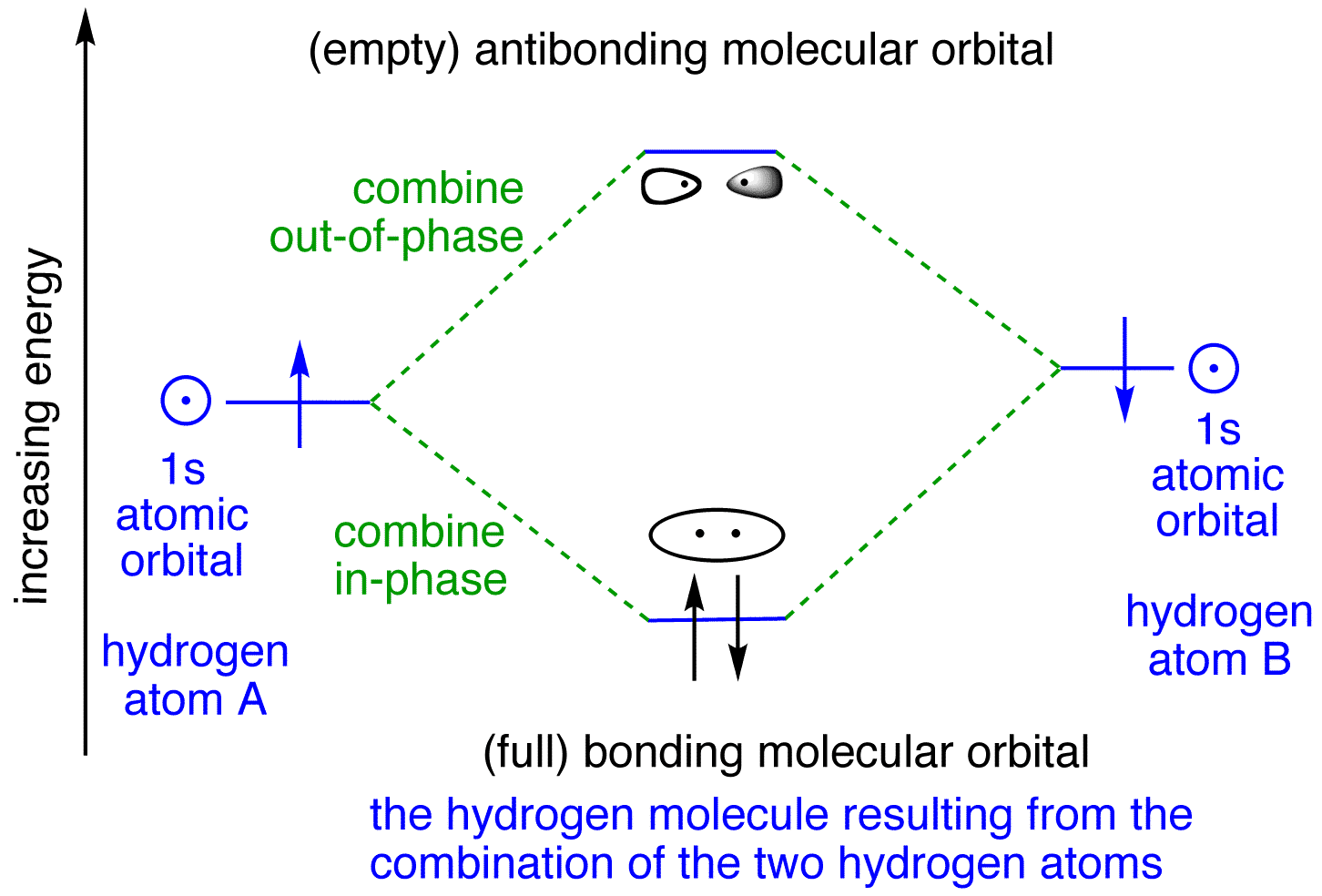
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H Molecular Orbitals for Larger Molecules 1. Determine point group of molecule (if linear, use D2h and C2v instead of D∞h or C∞v) 2. Assign x, y, z coordinates (z axis is principal axis; if non-linear, y axes of outer atoms point to central atom)3. Find the characters of the reducible representationfor the combination of This animation explains the water molecule through the Molecular Orbitals TheoryFor more Chemistry animations check out the website:www.quimica3d.com
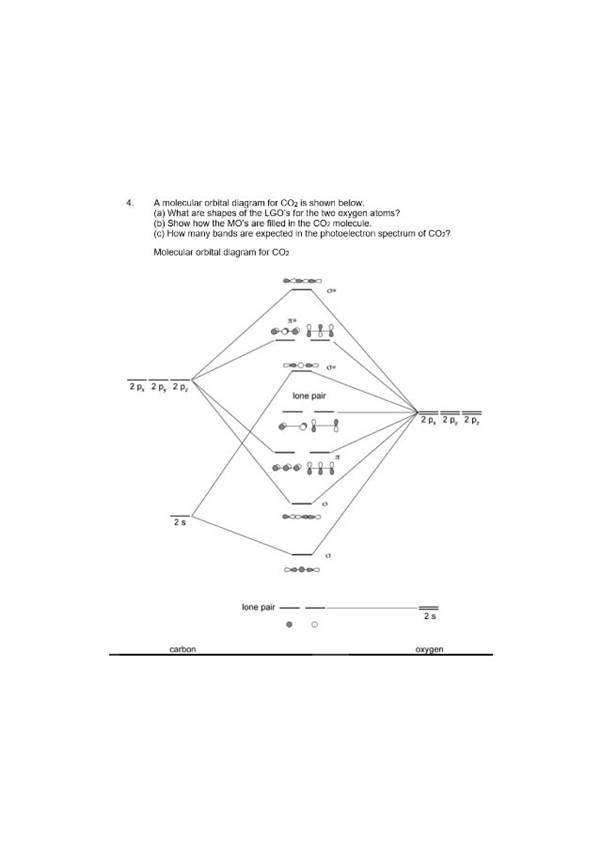
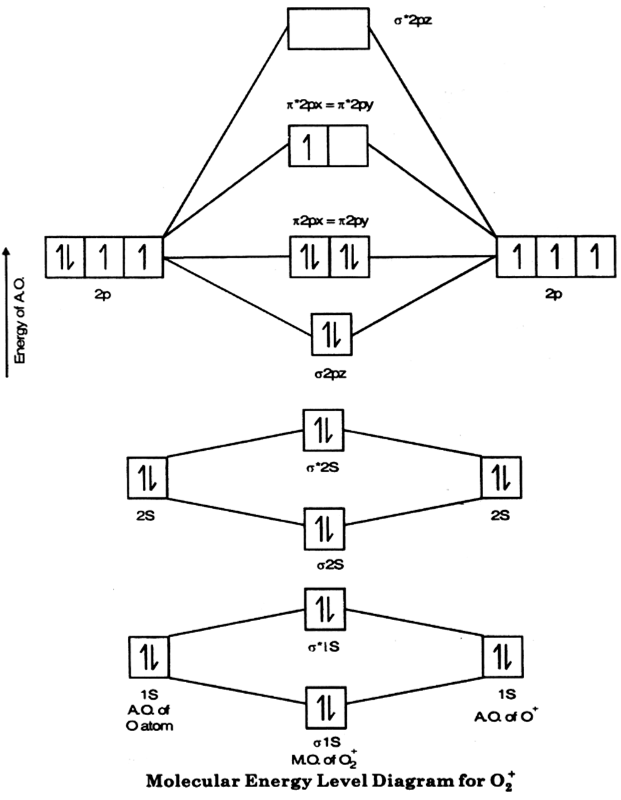
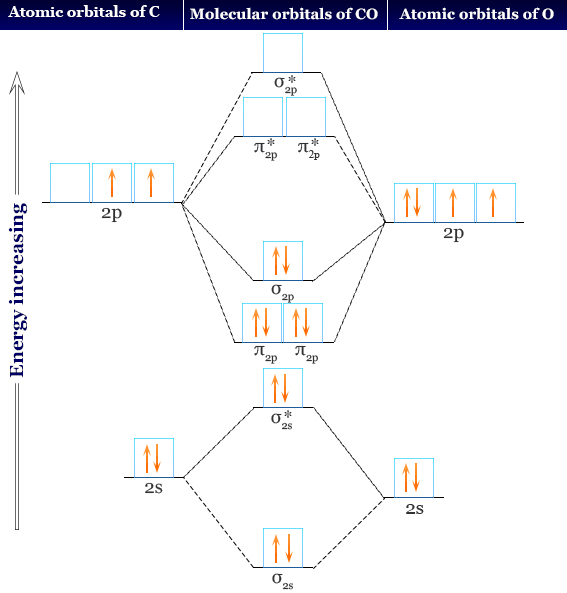
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
Formation of Molecular Orbitals. An atomic orbital is an electron wave; the waves of the two atomic orbitals may be in phase or out of phase. Suppose Ψ A and Ψ B represent the amplitude of the electron wave of the atomic orbitals of the two atoms A and B. Case 1: When the two waves are in phase so that they add up and amplitude of the wave is ...
Feb 02, 2022 · It is said that the 1s orbital of hydrogen overlaps and fuses with the 2p orbital of fluorine in a molecule of HF. According to Molecular Orbital Theory, the 2s orbital of F is non-bonding, and the 2pz orbital of F combines with 1s of H. HF Polarity. Polarity is yet another important topic of chemistry that we are going to discuss in this article.
Feb 01, 2022 · The molecular orbital diagram of ethane would be: The molecular orbital is formed from the combination of atomic orbitals, which must have nearly the same energy and are symmetrical about the molecular axis. To understand the MO diagram of ethane, we consider it as a homonuclear diatomic A2 molecule.
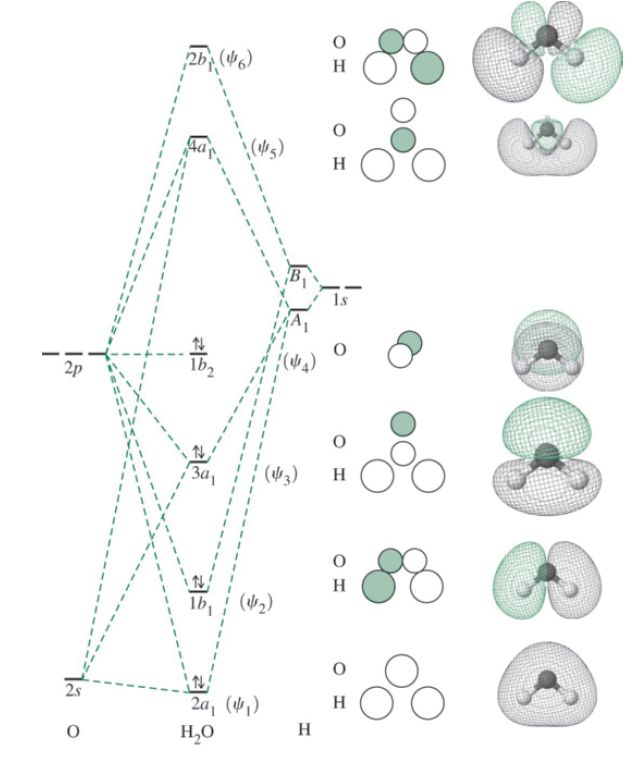
Molecular Orbitals for Water (H 2 O) The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2(2a 1) 2(1b 2) 2(3a 1) 2(1b 1) 2 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set (experimental data is given in [1289]). They are set out with the lowest
Molecular Orbitals for Water (H 2 O) The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1 a1) 2 (2 a1) 2 (1 b2) 2 (3 a1) 2 (1 b1) 2 (4a 1) 0 (2b 2) 0 (3b 2) 0 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. b (experimental data is given in [ 1289 ]).
In polyatomic molecules we can have more than two atoms combining, e.g. in case of beryllium hydride there are 3 atoms overlapping simultaneously. So in this...
The head-to-head overlap giving σ molecular orbitals results in greater overlap, making its bonding molecular orbital the most stable and lowest energy, while the σ* antibonding is least stable and has the highest energy (Figure 9.24 "Molecular orbital energy diagram for homonuclear diatomic molecules made from atoms of atomic number 8-10").
Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
We will walk through the steps below to construct the molecular orbital diagram of water. Preliminary Steps Step 1. Find the point group of the molecule and assign Cartesian coordinates so that z is the principle axis. The H 2 O molecule is bent and its point group is C 2 v. The z axis is collinear with principle axis, the C 2 axis.
H20 Molecular Orbital Diagram. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals. General procedure for simple molecules that contain a central atom: build group orbitals using the outer atoms, then interact the group orbitals.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Chemical bonding of H2O - WikipediaChemical bonding of H2O - Wikipedia
Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. creates bonds from overlap of atomic orbitals ( s, p, d …) and hybrid orbitals ( sp, sp2, sp3 …) combines atomic orbitals to form molecular orbitals (σ, σ*, π, π*) forms σ or π bonds.
For two equivalent bonds (as the O-H bonds here are expected to be) with a bond angle θ, the hybridization of the localized bond orbital is given by s p λ where. λ = − 1 cos. . θ. Applying this to the known bond angle of water, we find that the O-H bond orbitals are ≈ s p 4, that is, 80% p and 20% s. So they are slightly more p in ...
Here, using density functional theory and post-Hartree-Fock methods, we reveal two hydrogen bonding molecular orbitals crossing the hydrogen-bond's O and H atoms in the water dimer. Energy ...



















0 Response to "40 water molecular orbital diagram"
Post a Comment