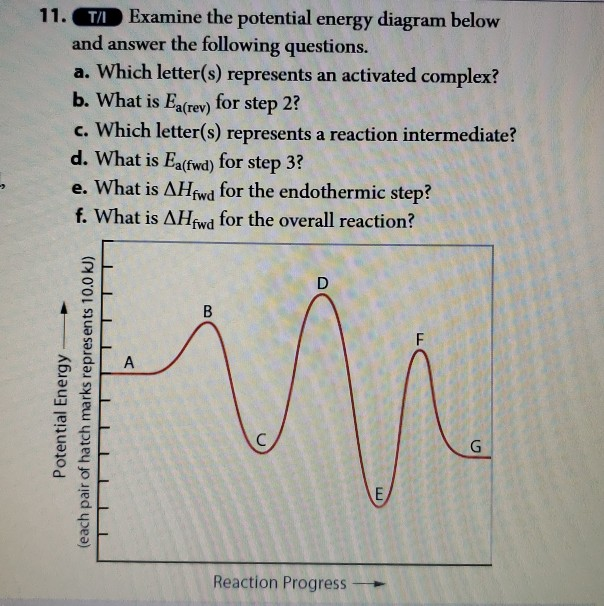
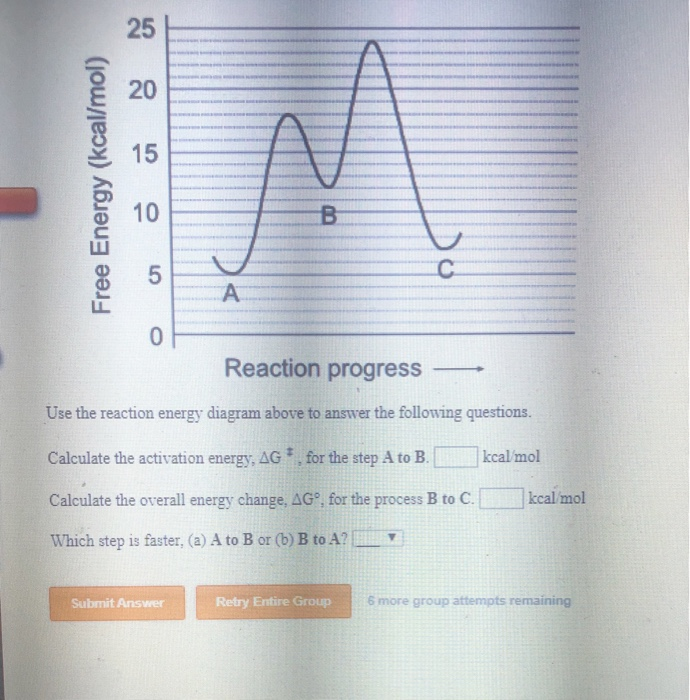
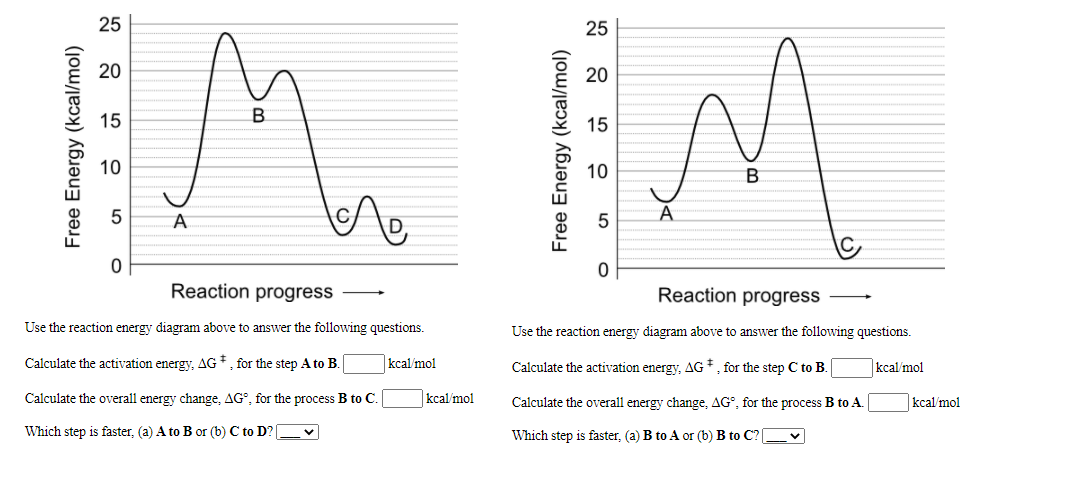
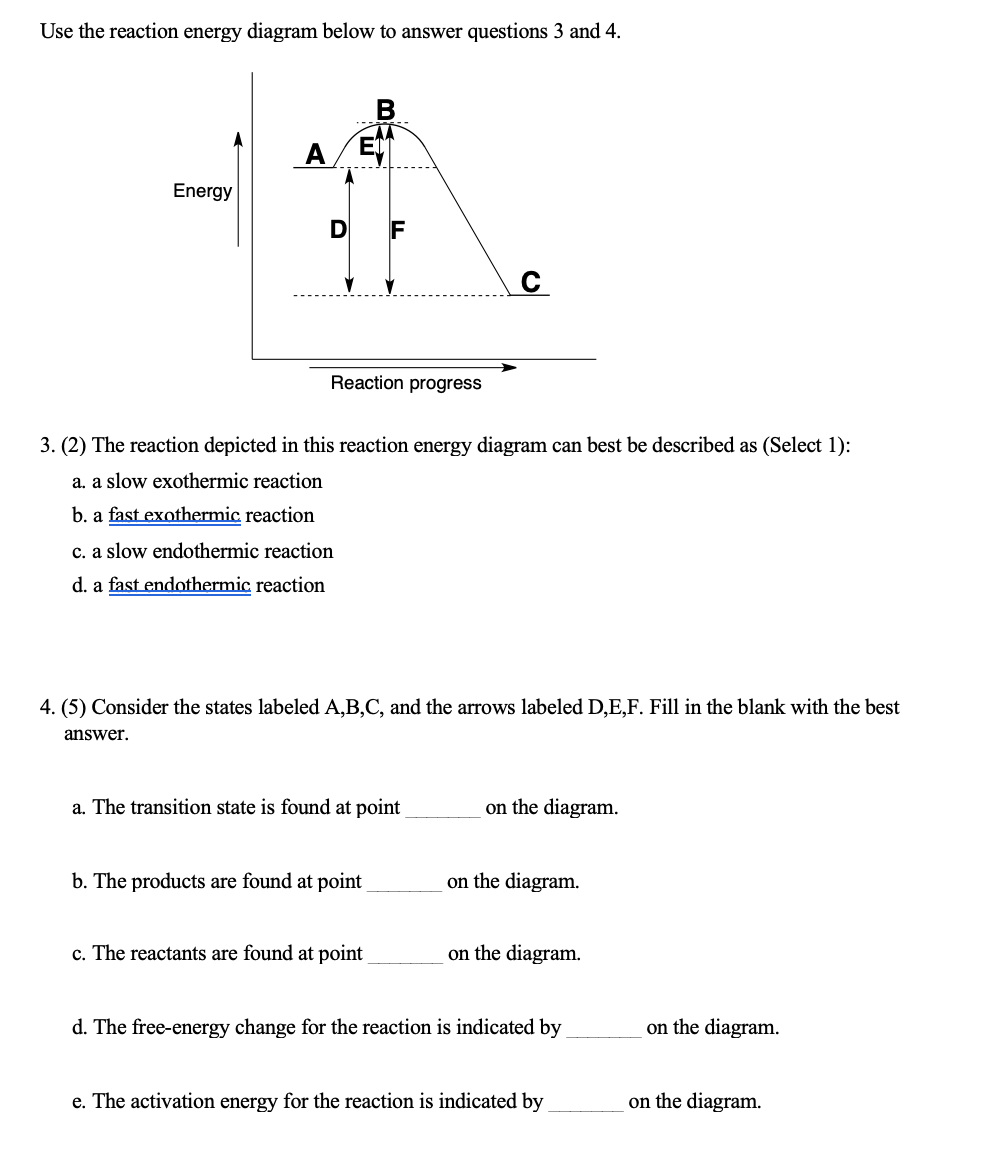
42 use the reaction energy diagram above to answer the following questions.
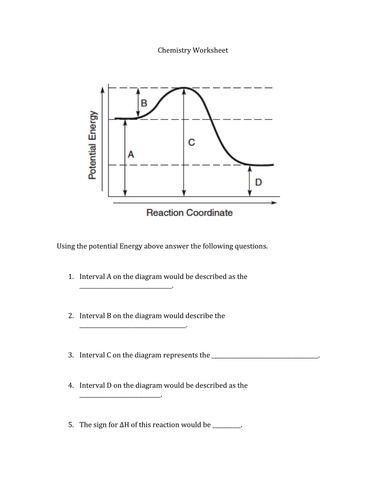
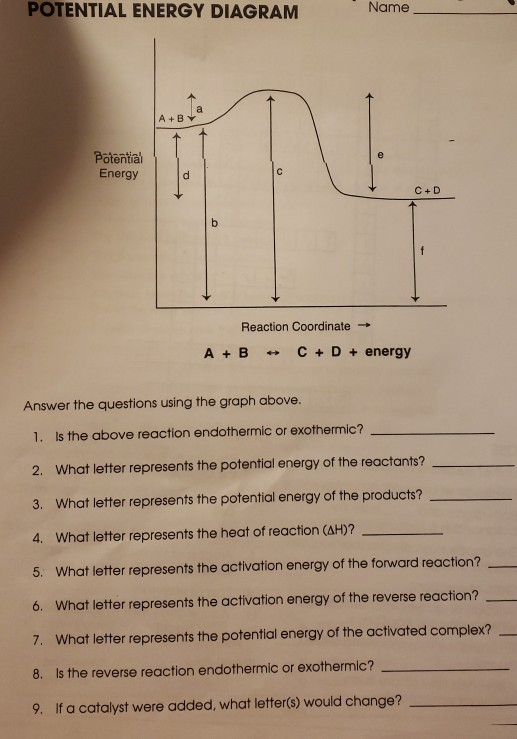
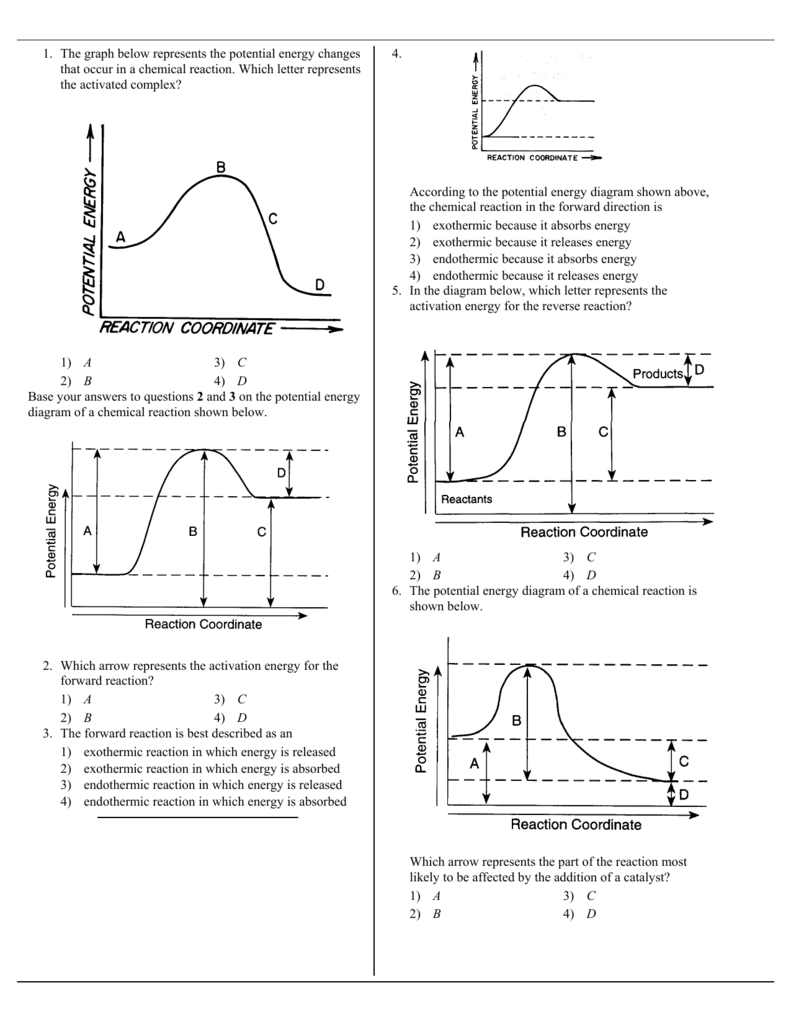
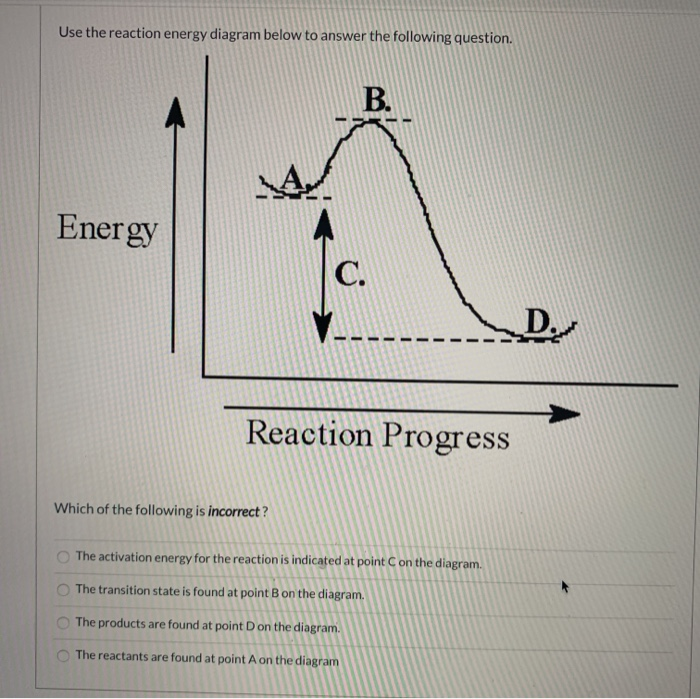
USE THE POTENTIAL ENERGY DIAGRAM TO ANSWER THE QUESTIONS BELOW: 1. ... for the reverse reaction on the graph above. 16. ... 20. Use the following Potential Energy Diagram to answer the questions below: 0 20 40 60 80 100 Progress of Reaction AB + C ABC A + BC View Notes - Potential E diagrams WS from CHEM 1001 at York University. Worksheet: Potential Energy Diagrams Use the following Potential Energy Diagram to answer the questions below: 1. Is the
Gaseous hydrogen and fluorine combine in the reaction above to form hydrogen fluoride with an enthalpy change of -540 kJ. What is the value of the heat of formation of HF(g)? (A) -1,080 kJ/mol (B) -270 kJ/mol (C) 270 kJ/mol (D) 540 kJ/mol Use the following information to answer questions 20-23. When calcium chloride (CaCl 2
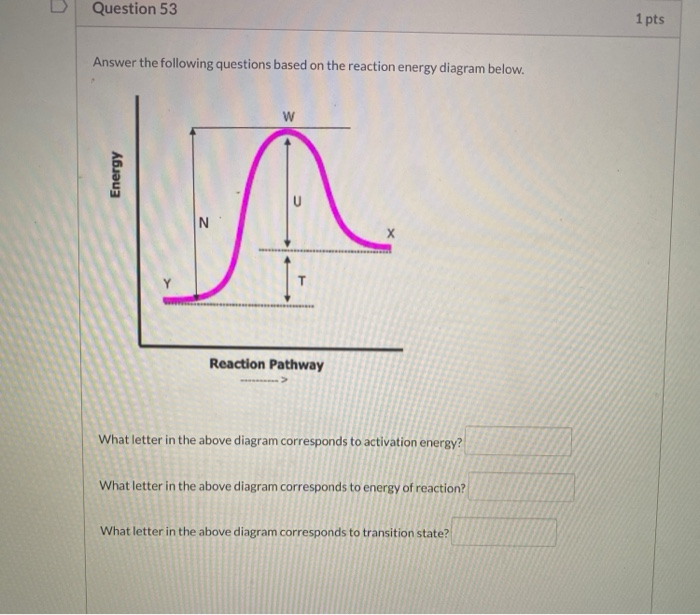
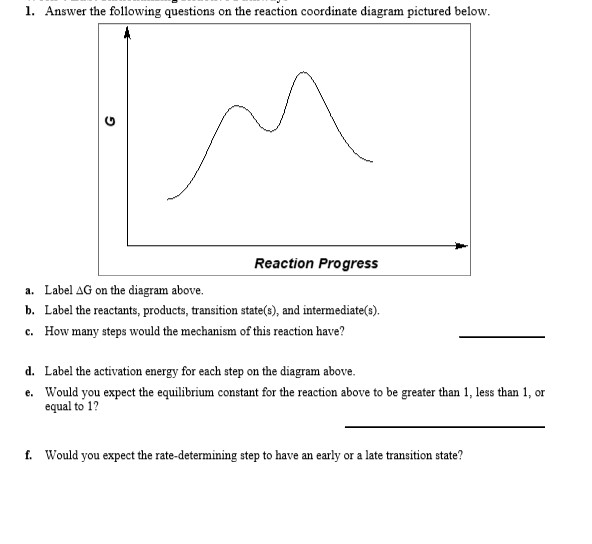
Use the reaction energy diagram above to answer the following questions.
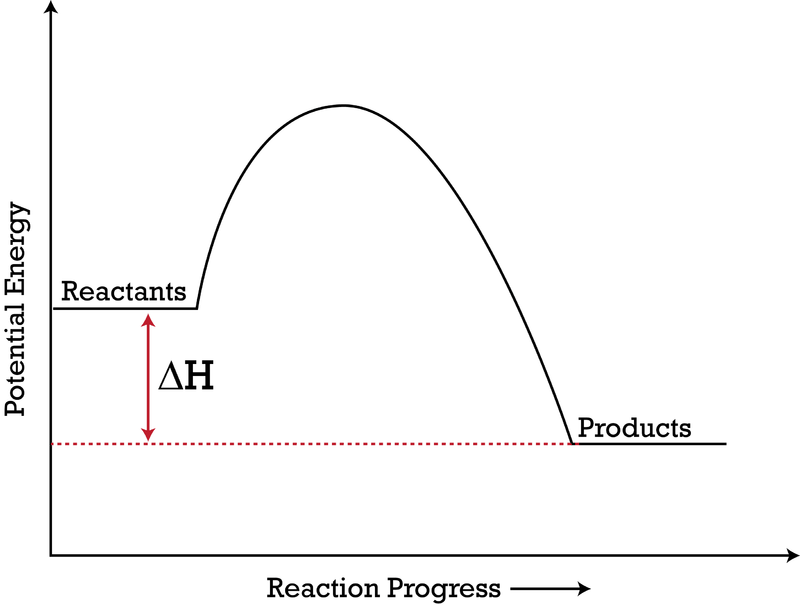
This reaction is represented by the following equation. Ti (s) + O 2 (g) → TiO 2 (s) + 944.0 kJ 20. The formation of titanium (IV) oxide is i , and the reactants have ii potential energy than the products. (K1E, S1A) The statement above is completed by the information in row Row i ii A. endothermic higher B. endothermic lower C. exothermic ... only way to make the reaction go faster is to add more enzymes. Answer the following questions from this section of reading. 4. In the diagram above, label A-E using the following vocabulary: active site, substrate, enzyme, glucose and fructose. 5. What is the substrate in the diagram above? The substrate in the diagram above is sucrose. 6. Answer: a . Explanation: Activation energy is the minimum amount of energy that must be overcome before a reaction will proceed to product. In the energy diagram, the thick line represent the uncatalyzed path while the broken line line indicates the catalyzed path.
Use the reaction energy diagram above to answer the following questions.. Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG, for the step A to B. kcal/mol. Calculate the overall energy change, ΔG°, for the process B to C. kcal/mol. Which step is faster, (a) A to B or (b) C to B 2) Name the following compound (3 pts.) OH Part 2. Use the reaction energy diagram to answer the following questions OH Products 3) What type of substitution reaction could be represented by the above reaction energy diagram? (3pts.) Topic 5.6 Reaction Energy Profile Worksheet Use the following potential energy diagram to answer questions 1-7 below: 1. Is the overall reaction, as shown, exothermic or endothermic? 2. Which letter represents the activation energy for the forward reaction? 3. Which letter represents the activation energy for the reverse reaction? 4. 6. Use your knowledge of kinetics to answer the following questions. Justify your answers. (a) Potential Energy 1 2 Reaction Coordinate The two lines in the diagram above show different reaction pathways for the same reaction. Which of the two lines shows the reaction when a catalyst has been added? (b) Fractions of Molecules 1 2 Energy Which ...
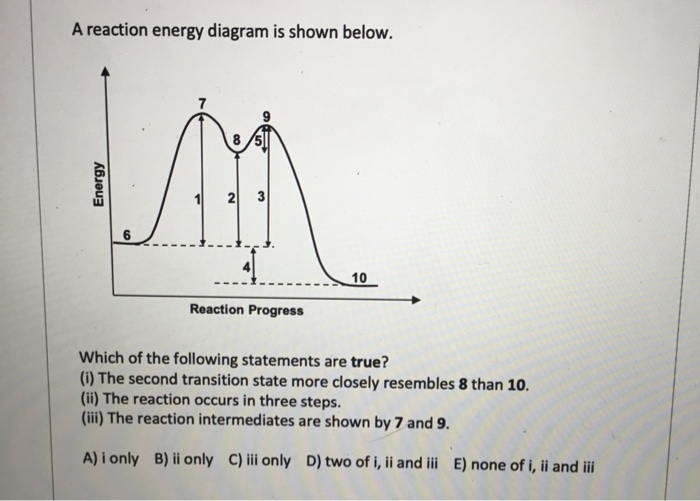
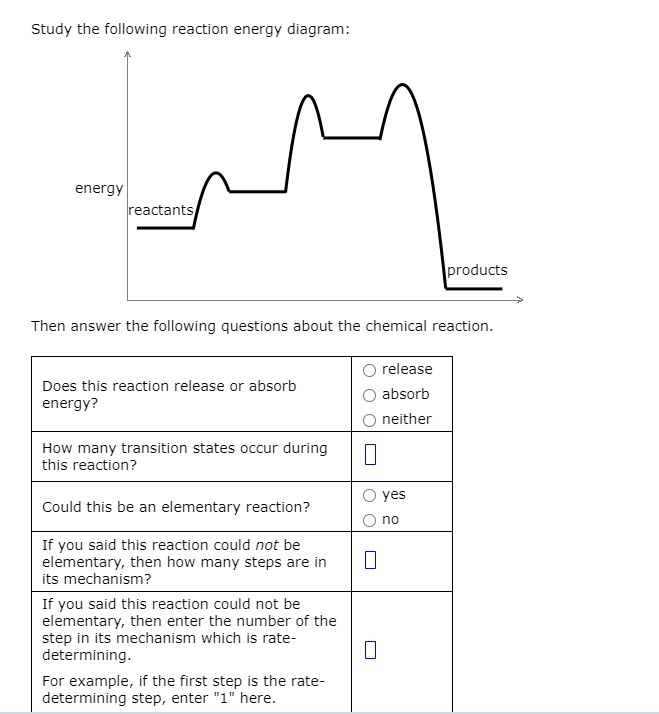
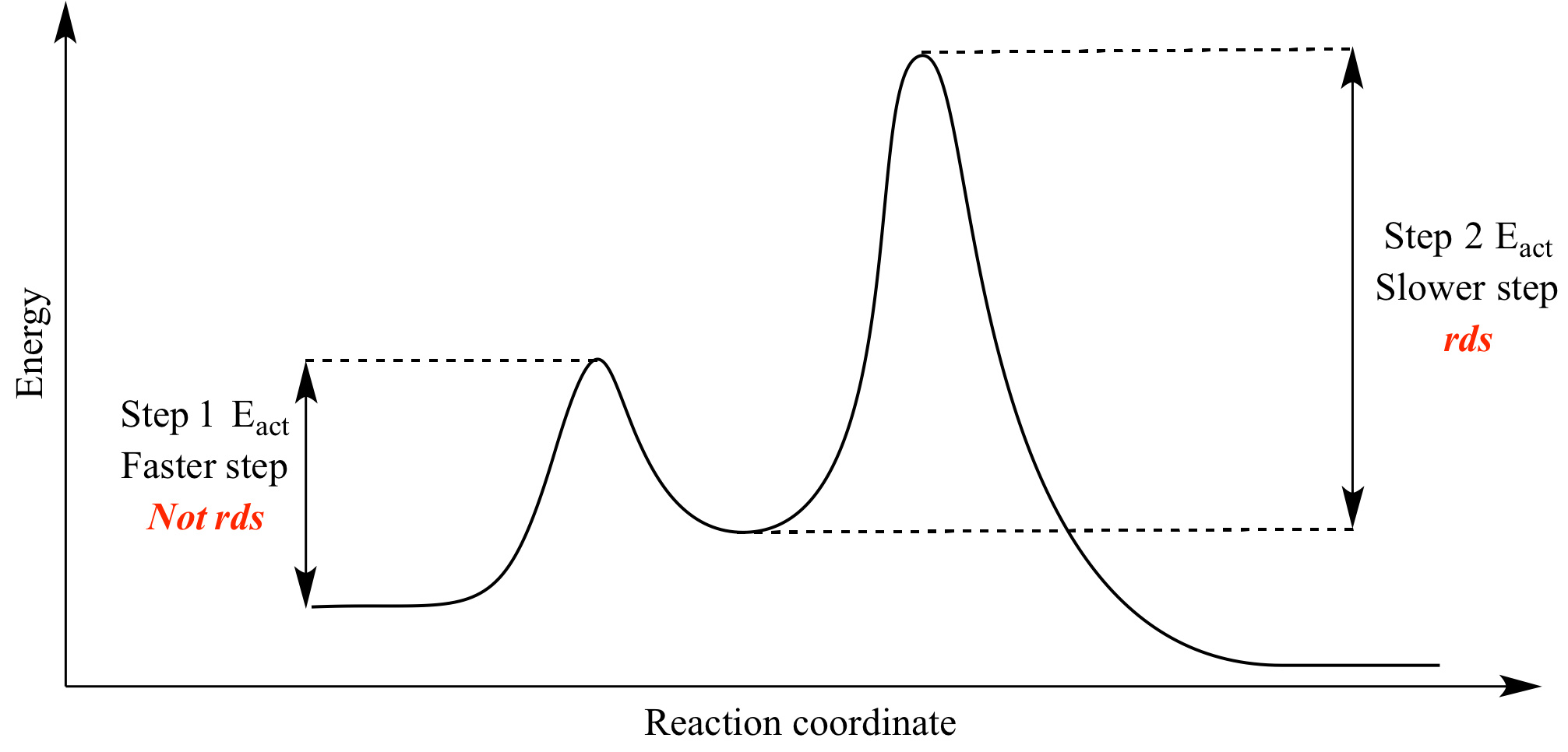
Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG, for the step A to B. kcal/mol. Calculate the overall energy change, ΔG°, for the process B to C. kcal/mol. Which step is faster, (a) A to B or (b) C to D? Q2. Use the reaction energy diagram above to answer the following questions. Consider the reaction energy diagram shown, and answer the following questions: How many steps are involved in the reaction? 2 Which step is the rate-determining step? 1 st Label the transition state(s) and intermediate(s) on the graph. Potential Energy Diagrams Problem Set ╪Use the following potential energy diagram to answer questions 1-8 below: F╪ 1. exothermic Is the overall reaction, as shown, exothermic or endothermic? 2. B. Which letter represents the activation energy for the forward reaction? 3. C. Which letter represents the activation energy for the reverse ... Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, delta G^for the step C to B kcal/mol Calculate the overall energy change, delta G degree, for the process B to A. kcal/mol. Which step is faster, (a) B to A or (b) B to C? Show full question. Answer. + 20. Watch.
Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. Problem: Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG for the step C to B _____ kcal/mol Calculate the overall energy change, ΔG°, for the process B to A. _____kcal/mol. 25 E 20 é 15 C D 0 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, Î G , for the step A to B. Calculate the overall energy change, AGo, for the process B to C. Friction would do negative work and thus remove mechanical energy from the falling ball. Use the following diagram to answer questions #3 - #5. Neglect the effect of resistance forces. 3. As the object moves from point A to point D across the surface, the sum of its gravitational potential and kinetic energies ____.
Questions; mathematics. 1. Use the table to answer the question: Bond Bond Energy H-H 432 Br-Br 193 I-I 149 H-Br 363 H-I 295 Hydrogen bromide breaks down into diatomic hydrogen and bromine via the following reaction: 2HBr → H2 + Br2 Hydrogen iodide is formed from diatomic hydrogen and iodine via the following reaction: H2 + I2 → 2HI
Use the potential energy diagram below to answer the following question The potential energy of the reactants is ____kJ, while the potential energy of the products is _____. ( straight, wayyyyyyyyyyy uphill, then tiny downhill, then straight )
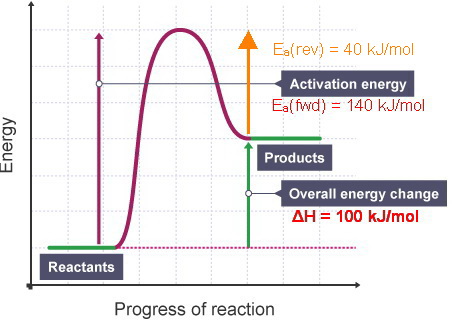
The diagram represents a spontaneous reaction. Use the diagram to answer the questions below. a. Is the reaction endothermic or exothermic? b. What is the activation energy of the reaction?
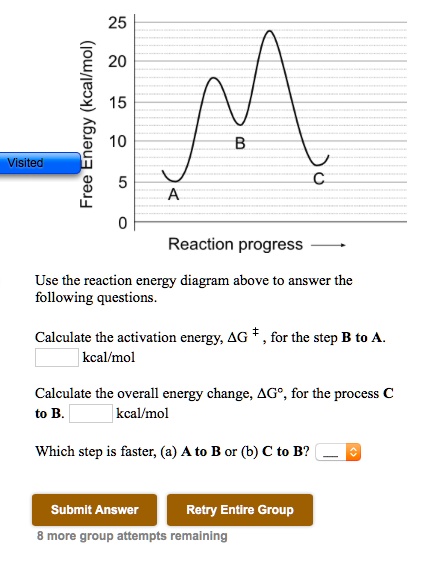
25 Free Energy (kcal/mol) 20 15 10 B A C 0 Reaction progress - Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG * , for the step B to A. kcal/mol Calculate the overall energy change, AG, for the process C to B. kcal/mol Which step is faster, (a) A to B or (b) C to B?
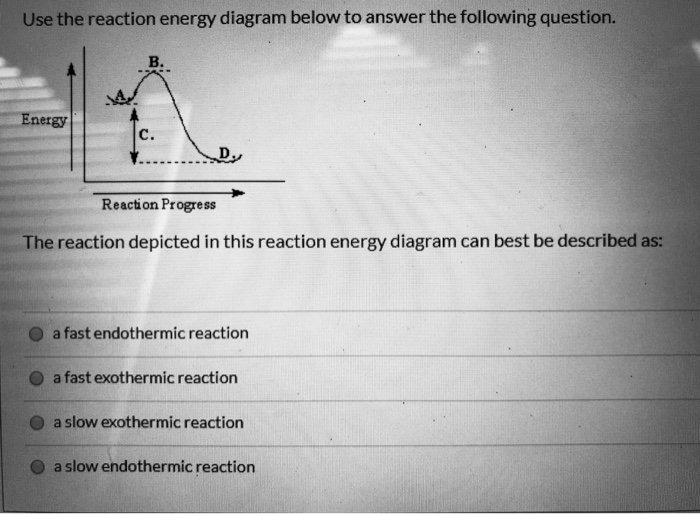
The reaction is exothermic. The reaction is slow at 25°C; however, a catalyst will cause the reaction to proceed faster. (e) Using the axes provided below, draw the complete potential-energy diagram for both the catalyzed and uncatalyzed reactions. Clearly label the curve that represents the catalyzed reaction.
Reaction Il is endothermic, and the activation energy of reaction I is greater than that of reaction O Reaction Pathway (a) Complete the potential-energy diagram for reaction Il on the graph above.. (b) For reaction I, predict how each of the following is affected as the temperature is increased by 200C. Explain the basis for each prediction.
Free Energy (kcal/mol) B. 25 20 15 10 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG, for the step B to C. kcal/mol Calculate the overall energy change, AG°, for the process A to B. kcal/mol Which step is faster, (a) C to B or (b) B to A? Free Energy (kcal/mol) B.
Kinetics - Free Response Sample Questions 2005 B Answer the following questions related to the kinetics of chemical reactions. I- (aq) + ClO- (aq) O H- o IO (aq) + Cl- (aq) Iodide ion, I-, is oxidized to hypoiodite ion, IO-, by hypochlorite, ClO-, in basic solution according to the equation above.
Transcribed image text: 25 20 15 Free Energy (kcal/mol) 10 B 5 A 0 Reaction progress Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, AG +, for the step C to B. kcal/mol Calculate the overall energy change, AG®, for the process B to A. kcal/mol Which step is faster, (a) B to A or (b) B to C?
Use the reaction energy diagram above to answer the following questions. Calculate the activation energy, ΔG, for the step B to C. kcal/mol Calculate the overall energy change, ΔG°, for the process A to B. kcal/mol Which step is faster, (a) C to B or (b) B to A? For the reaction below: a. Estimate the gas phase enthalpy change using bond.
Use the reaction energy diagram above to answer the following questions. a. Calculate the activation energy, ΔG, for the step C to B. b. Calculate the overall energy change, ΔG°, for the process B to D. c. Which step is faster, (a) B to A or (b) A to B? Question: Use the reaction energy diagram above to answer the following questions. a.
Answer: a . Explanation: Activation energy is the minimum amount of energy that must be overcome before a reaction will proceed to product. In the energy diagram, the thick line represent the uncatalyzed path while the broken line line indicates the catalyzed path.
only way to make the reaction go faster is to add more enzymes. Answer the following questions from this section of reading. 4. In the diagram above, label A-E using the following vocabulary: active site, substrate, enzyme, glucose and fructose. 5. What is the substrate in the diagram above? The substrate in the diagram above is sucrose. 6.
This reaction is represented by the following equation. Ti (s) + O 2 (g) → TiO 2 (s) + 944.0 kJ 20. The formation of titanium (IV) oxide is i , and the reactants have ii potential energy than the products. (K1E, S1A) The statement above is completed by the information in row Row i ii A. endothermic higher B. endothermic lower C. exothermic ...




















0 Response to "42 use the reaction energy diagram above to answer the following questions."
Post a Comment