43 methane molecular orbital diagram
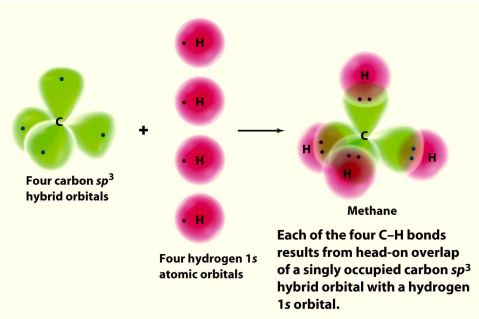
The shape of methane When sp3orbitals are formed, they arrange themselves so that they are as far apart as possible. That is a tetrahedral arrangement, with an angle of 109.5°. Nothing changes in terms of the shape when the hydrogen atoms combine with the carbon, and so the methane molecule is also tetrahedral with 109.5° bond angles. Ethane, C2H6 The resulting angle between orbitals is 109.5°. Four Hydrogen atoms bond with Carbon to give methane. Hydrogen’s spherical 1s orbital merges with one of Carbon’s sp 3 orbitals to form a new molecular bonding orbital with Hydrogen’s nucleus embedded in it. The bond produced from the overlap of the two atomic orbitals is called a sigma ...
Please explain me the orbital diagram and electron Dot structure of ccl4, h2o, nh3,ch4. why does graphite have high melting or boiling point if their bonding is weak. give one property of hydrogen chloride which agrees with it being a covalent compound. A covalent hydrocarbon molecule having four single covalent bond.
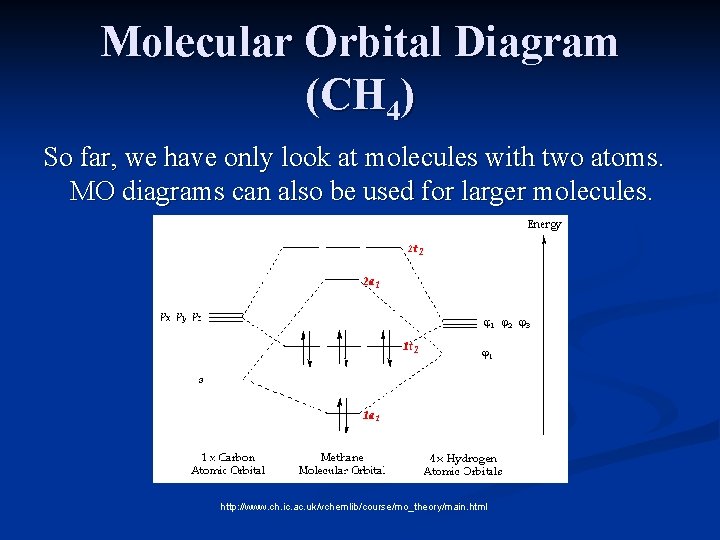
Methane molecular orbital diagram
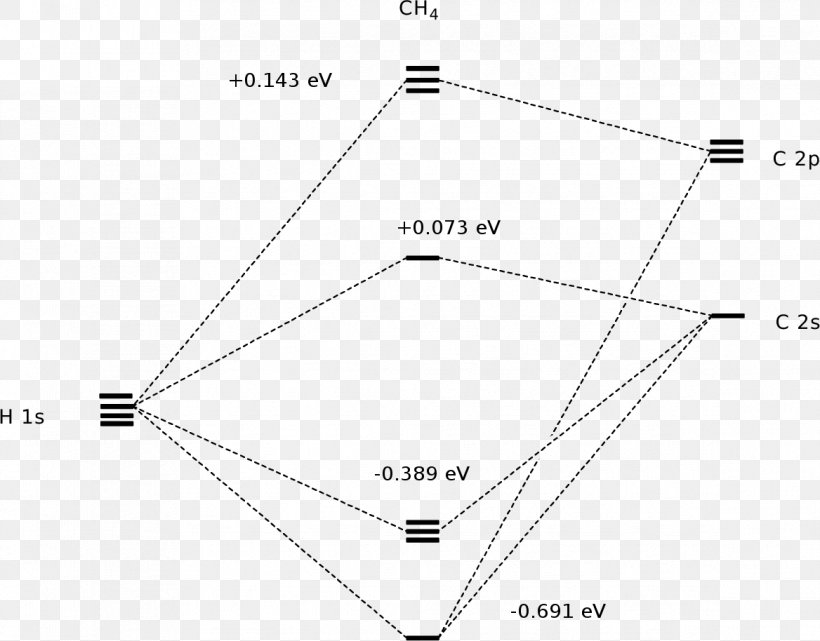
A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. ) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two. Ethene: The simplest alkene is ethene. Ethane. • As molecules get bigger constructing the molecular orbitals. becomes more challenging. • Insights into bonding of larger molecules can be attained by. combining fragments with well defined MO's... through orbital. mixing. • In this manner, ethane can be constructed from MO's of two. pyramidal CH3 groups. A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals.
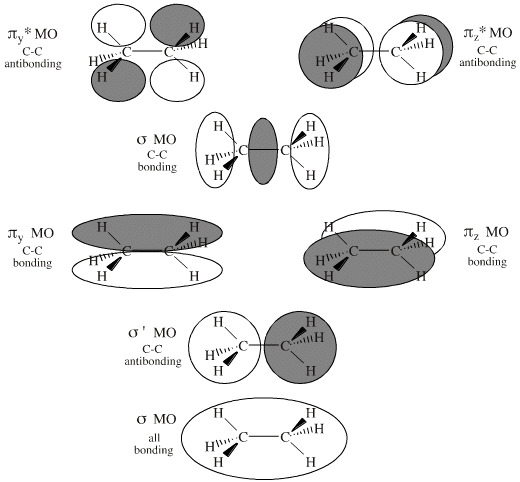
Methane molecular orbital diagram. square planar methane. With no lone pairs, the electronic and molecular geometry are the same. Technique for constructing LGO’s: I Draw Lewis structure and assign VSEPR geometry (already done above) II Assign a point group to the molecular geometry III determine the central atom’s VB hybrid orbitals for the electronic geometry Methane has four valence molecular orbitals (bonding), consisting of one orbital with one nodal plane (lowest occupied) and three degenerate (equal energy) orbitals that do have a nodal plane. For the energy diagram and pictorial view of the orbitals - please see below: Ethane: Chapter 2. Alkanes and Cycloalkanes: Introduction to Hydrocarbons 2.1: Classes of Hydrocarbons molecules that are made up of carbon and hydrogen Methane's MOs have a topology similar to the AOs of carbon, but the structure can be very difficult to visualise, so the methane MO construction diagrams A, B and C (below) are shown with the AOs and MOs superimposed upon line structures of the methane.
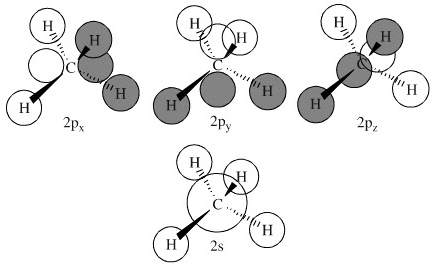
The three molecular orbitals (MOs) of methane in the ground electronic state (X 1 A 1 ), namely, the core MO, 1 a1 and the valence MOs, i.e. 2 a1 and three-fold energy degenerate MOs 1 t2, are studied in both coordinate space and momentum space. The diatomics N two and CO each had ten electrons to go into their eight molecular orbitals. And here, with methane, we have eight electrons, four coming in as the valence orbitals from the carbon atom, valence electrons from the carbon atom, rather, and four coming in as the four valence 1s electrons for the four hydrogens. Molecular orbital theory (MOT) for methane forms bonding molecular orbitals involving linear combinations of the unhybridized carbon 2s and 2p valence orbitals with the hydrogen 1s orbitals as shown in the graphic below. The plus and minus signs signify phase, not electrical charge. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals. Generate the Molecular Orbitals for CH4(Td), CH4(D4h) and Cyclopropane using diagram between the bonding MOs of square planar and tetrahedral CH4. The molecular orbital description of bonding in methane does several things for us.
e) Show the resulting molecular orbital energy level diagram for borane. f) Tetrahedral atoms are referred to as sp 3. What hybrid label would you give your boron with this geometry? Problem MO12.2. Suppose you wanted to use a hybridization approach to show a rough molecular orbital diagram for beryllium hydride, BeH 2. The three molecular orbitals (MOs) of methane in the ground electronic state (X(1)A(1)). namely, the core MO, 1a(1) and the valence MOs, i.e. 2a(1) and three-fold energy degenerate MOs 1t(2) are ... molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. It is a linear molecule. Chemistry 104 ... p-orbitals; 3p-orbitals; 3d-orbitals; 4f-orbitals; Compare shape and size of 1s, 2s and 2p orbitals; Molecular Orbitals. Hydrogen; Nitrogen; Fluorine; Ammonia; Methane; Ethylene (Ethene) Acetylene (Ethyne) Allene; Formaldehyde(Methanal) Acrolein; Carbon Monoxide; Hydrogen Fluoride; Allyl Anion; Butadiene; Benzene; Aromaticity of cyclic polyenes ...
The Shape of Methane When sp 3 orbitals are formed, they arrange themselves so that they are as far apart as possible. That is a tetrahedral arrangement, with an angle of 109.5°. Nothing changes in terms of the shape when the hydrogen atoms combine with the carbon, and so the methane molecule is also tetrahedral with 109.5° bond angles.
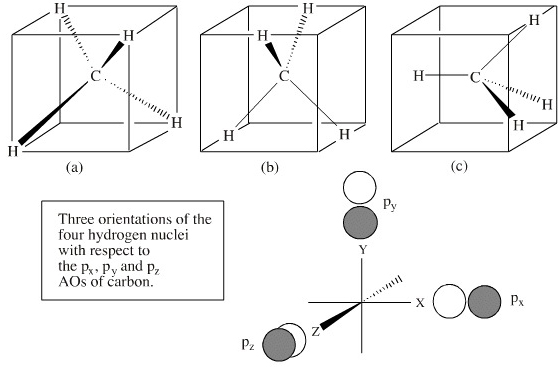
This in-class activity walks students through the preparation of a molecular-orbital diagram for methane in a square-planar environment. The students generate ligand-group orbitals (LGOs) for the set of 4 H(1s) orbitals and then interact these with carbon, ultimately finding that such a geometry is strongly disfavored because it does not maximize H/C bonding and leaves a lone pair on C.
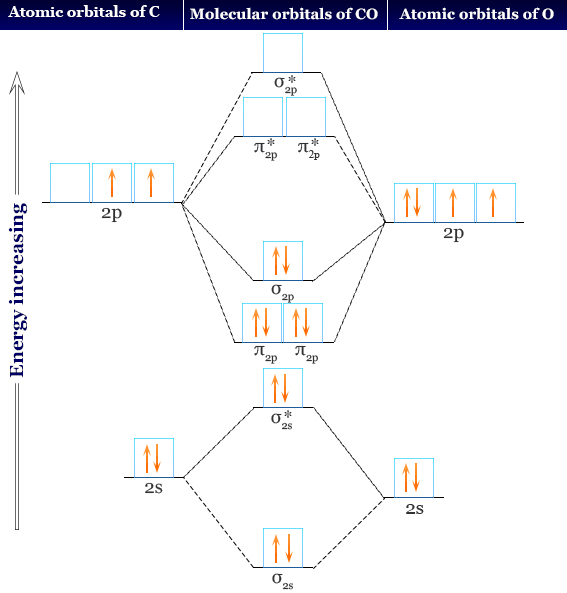
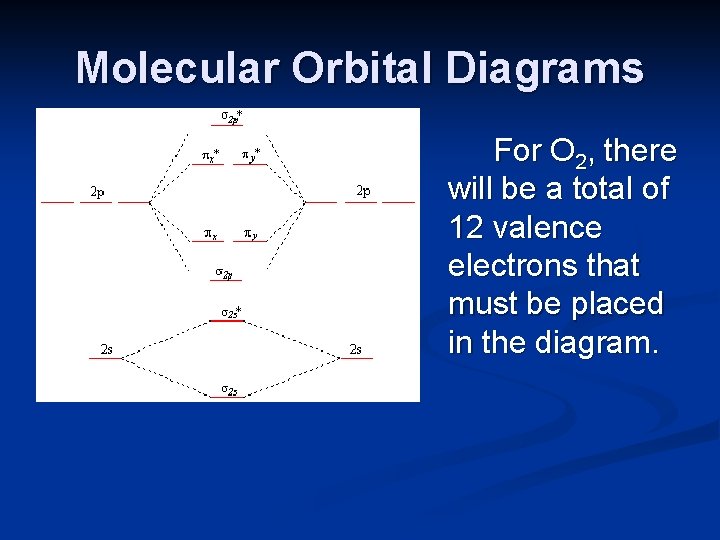
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
The bonds in a methane (CH4) molecule are formed by four separate but equivalent orbitals; a single 2s and three 2p orbitals of the carbon hybridize into four sp 3 orbitals. In the ammonia molecule (NH 3 ), 2s and 2p orbitals create four sp 3 hybrid orbitals, one of which is occupied by a lone pair of electrons.
The molecular orbital description of bonding in methane does several things for us. It should reconcile our valence-bond idea of electrons localized between carbon and hydrogen with the "delocalized" picture typical of the MO approach. It should tell us (quantitatively) about the energies of different electrons.
This animation explains the methane molecule through the Molecular Orbitals TheoryFor more Chemistry animations check out the youtube channel Lili Tosta: htt...
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining ... For simple polyatomic molecules with a "central atom" such as methane (CH
A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. 388) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two lowest energy molecular orbitals ...
Methane Molecular Orbitals. In the following model, the carbon atom is dark gray and the hydrogens are cyan. The hydrogen atoms are arbitrarily numbered. A molecular orbital will be displayed by pressing the appropriate button.The different phases of the molecular orbitals are colored red and blue and are separated by nodal surfaces at which electron density is zero.
A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. 388) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two lowest energy molecular orbitals ...
Question: How many bonding and antibonding molecular orbitals are there in methane, CH4? Bonding orbitals Zero One Two Three Four Antibonding orbitals Zero One Two Three Four . This problem has been solved! See the answer See the answer See the answer done loading. Help! I know nothing about antibonding...
Determining CH 4 molecular geometry should be easier. In methane, the four hybrid orbitals are located in such a manner so as to decrease the force of repulsion between them. Nonetheless, the four orbitals do repel each other and get placed at the corners of a tetrahedron. CH 4 has a tetrahedral shape. The sp 3 hybrid orbitals have a bond angle ...
Methane is a pentatomic, tetrahedral molecule. It is formed by combination of one carbon atom with 4 hydrogen atoms. In the molecule of methane, the carbon a...
Construct the molecular orbital diagram for dichlorine. x y z z y 3 x y z z y 4 Showing the p orbitals. Showing the s and p orbitals. ORBITALS AND MOLECULAR REPRESENTATION 11. CARBON ORBITALS Methane Ethane METHANE AND ETHANE C H H H H CH4 C C H H H H H H C2H6 1 2 Color conventions: Hydrogen atoms are shown in gray.
A Molecular Orbital Approach to Bonding in Methane methane (CH4) molecule . A molecular orbital diagram showing both the bonding and anti-bonding. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals.
Ethane. • As molecules get bigger constructing the molecular orbitals. becomes more challenging. • Insights into bonding of larger molecules can be attained by. combining fragments with well defined MO's... through orbital. mixing. • In this manner, ethane can be constructed from MO's of two. pyramidal CH3 groups.
A molecular orbital diagram showing both the bonding and anti‐bonding molecular energy levels is provided below. (McQuarrie & Simon, Physical Chemistry: A Molecular Approach, p. ) Methane has eight valence electrons, so according to the aufbau and Pauli exclusion principles the two. Ethene: The simplest alkene is ethene.
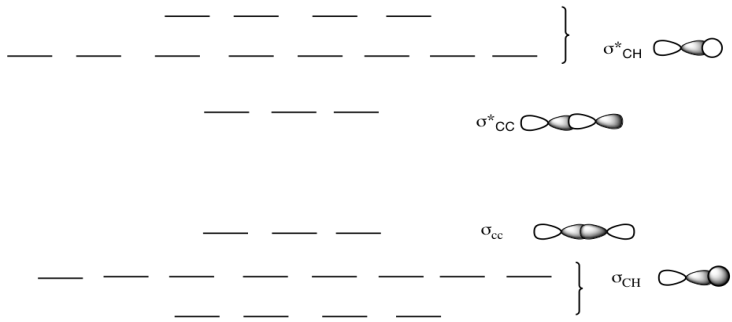






















0 Response to "43 methane molecular orbital diagram"
Post a Comment