44 adiabatic expansion pv diagram
The PV diagram models the relationship between pressure (P) and volume (V) for an ideal gas . The fundamental thermodynamic processes modelled on PV diagrams (isochoric, isobaric, and isothermal processes) all follow the ideal gas law except for adiabatic processes—which will be discussed in... When an ideal gas expands adiabatically and reversibly, the change in entropy of the gas and the surroundings should both be 0 since dq=0 for an adiabatic expansion. When the same occurs but instead it is not reversible and expands into a vacuum, is the entropy change for the gad and the surroundings still 0? Also is the first statement correct? Thank you in advance.
This physics video tutorial explains the concept of the first law of thermodynamics. It shows you how to solve problems associated with PV diagrams...
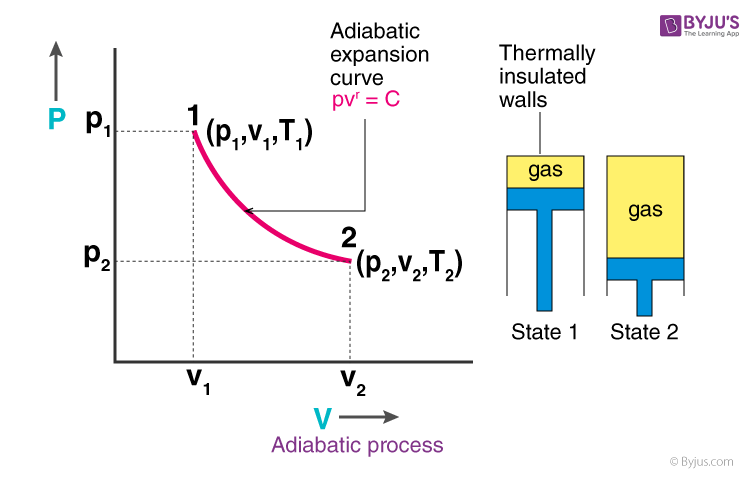
Adiabatic expansion pv diagram
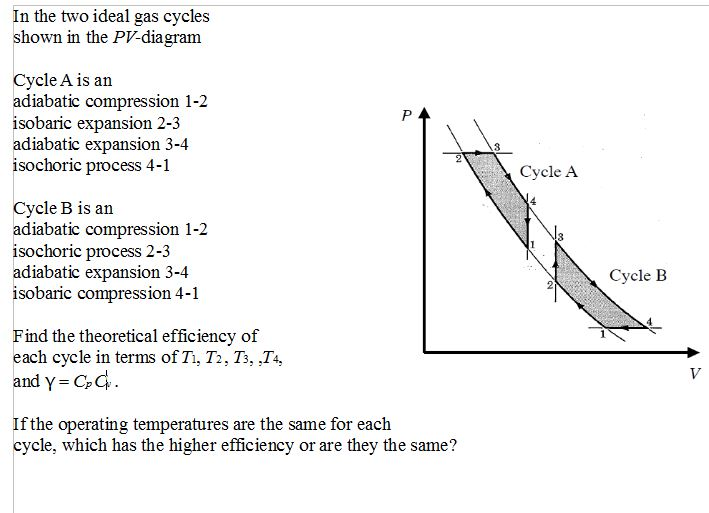
Lesson 42c: PV Diagrams. From the last section, you were probably wondering what happens when we do something like add heat. During an isobaric process the gas is heated and expands from 0.25 m3 to 0.55 m3. Determine the work done by the gas on the cylinder, and sketch a PV diagram of the... Can anyone explain why it's drawn like that? The operation of the Otto cycle on a p-V diagram is shown in Fig. 5.3. The cycle comprises four processes: adiabatic compression 1 → 2, isochoric heat addition 2 → 3, adiabatic expansion 3 → 4, and isochoric heat removal 4 → 1. Thus, for the Otto cycle, we have V 2 = V 3, V 4 = V 1, CR = V 1 /V 3, and PR = p 3 /p 1.
Adiabatic expansion pv diagram. An adiabatic process is a reversible constant entropy process for an ideal gas without heat transfer, following the relationship. Because d Q = 0 for an infinitesimal adiabatic process, the first law requires for a gas undergoing a quasi-static adiabatic process that. then expands adiabatically to its original pressure and fi- nally is compressed isobarically to its original volume. (a) Draw a PV diagram of this cycle. (b) Determine the vol- ume at the end of the adiabatic expansion. Find (c) the temperature of the gas at the start of the adiabatic expan- sion and (d)... 2.11 Adiabatic changes - Poisson equations. The next sections will discuss the theoretical background for describing experiments performed under various In this section we discuss adiabatic processes, i.e. processes without heat transfer to or from the surrounding. Such a condition can of course be... CPS question This pV-diagram shows two ways to take a system from state a (at lower left) to state c (at upper right) Thermodynamic Processes: •Adiabatic: no heat transfer (by insulation or by very fast process). Q=0 → U2 - U1 = -W •Isochoric: constant volume process (no work done).
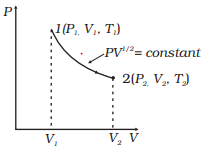
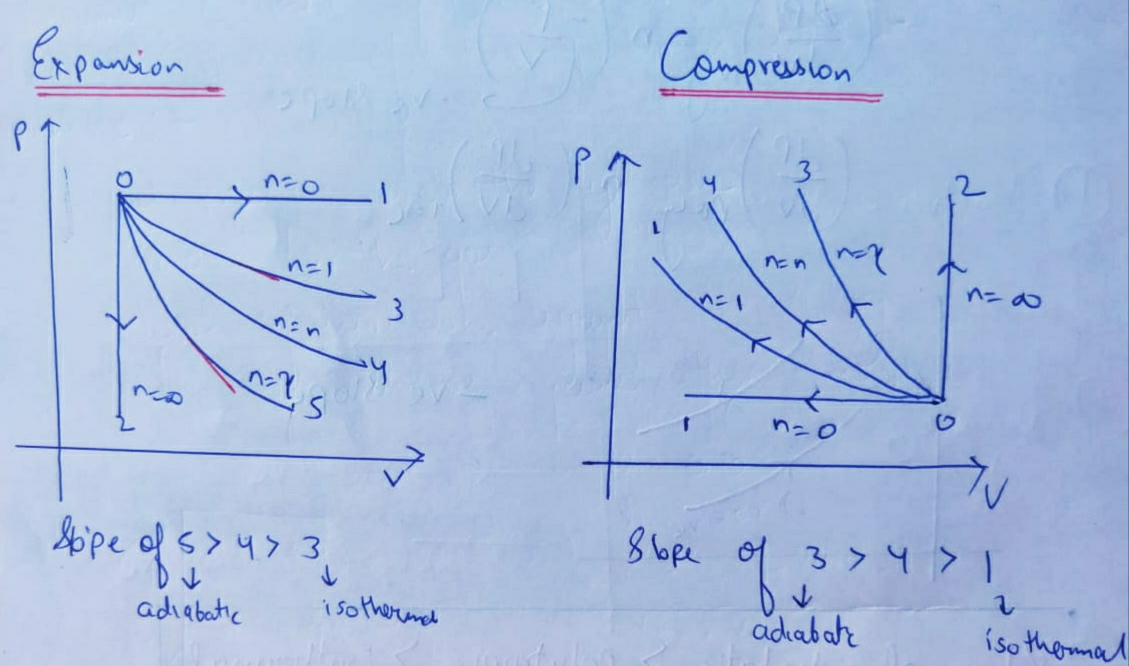
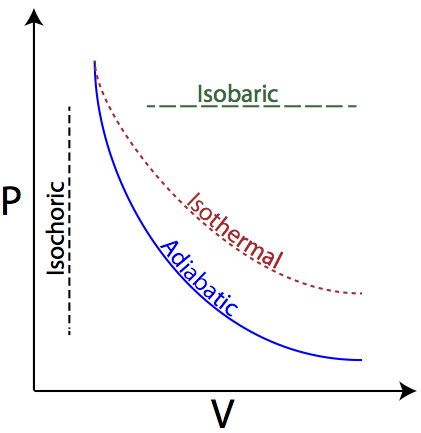
Playing around with this: [http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/adiab.html#c3](http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/adiab.html#c3) ​ and setting γ = 1.33 for steam, I get PV\^γ=143462 ​ Is there some place to look up this constant for various substances? Adiabatic means when no heat exchange occurs during expansion between system and surrounding and the temperature is no longer held constant. This equation shows the relationship between PV and is useful only when it applies to ideal gas and reversible adiabatic change. V, T ) can change during an adiabatic process. We get PiVi =. Pf V f 15/08/07 (Q=0) Assume an ideal gas is in an equilibrium state and so PV = nRT is valid. The adiabatic curve in the PV diagram depends on For a monatomic gas CV=3/2R, CP=5/2R and = 5/3 For a diatomic CV=5/2R, CP=7/2R... Define adiabatic expansion of an ideal gas. Demonstrate the qualitative difference between adiabatic and isothermal expansions. A reversible adiabatic expansion of an ideal gas is represented on the pV diagram of (Figure). The slope of the curve at any point is.
Get Adiabatic Expansion essential facts. View Videos or join the Adiabatic Expansion discussion. Add Adiabatic Expansion to your We can solve for the temperature of the compressed gas in the engine cylinder as well, using the ideal gas law, PV = nRT (n is amount of gas in moles and R the gas... Adiabatic exapansion and compression. The P(V) relation for an adiabatic process in an ideal gas. Physclips provides multimedia education in introductory physics (mechanics) at different levels. How does pressure vary with volume change during adiabatic expansion and compression in an ideal gas? Learn Adiabatic Process topic of Physics in details explained by subject experts on vedantu.com. Register free for online tutoring session to clear your Adiabatic- Reversible and Irreversible Process. There are four types of process in a thermodynamic system, which are shown via an image below The pV-diagram for the isothermal process is shown below. Now we consider an adiabatic process, with the same starting conditions and the same final. An expansion takes energy out of the gas as it does work on the environment. This tends to lower the temperature of the gas.
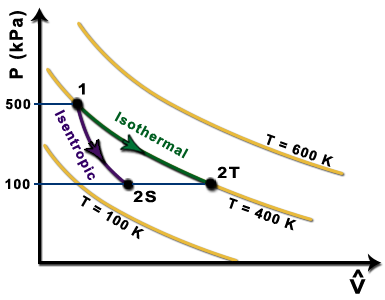
pV γ is constant along a reversible adiabat. Recall, for an isothermal process T = constant ⇒ pV = constant. p p1 isotherm. pV=constant pVγ=const. p2 adiabat. V2adiabat < V2isotherm because the gas. cools during reversible. adiabatic expansion.
I just want to share some interesting reads for the Nerds here that want to know what will go on in the next year or two. I think all major energy analysts are under expecting the massive growth in the next two years. Historically we have about 29% Compound annual growth rate for solar. The last two years underperformed at about 24% each. I think this is about to change and here is why: [https://www.pv-magazine.com/magazine-archive/whats-next-for-polysilicon/](https://www.pv-magazine.com/maga...
Assuming an initial temperature of 900 degrees Rankine, an initial pressure of 215 PSIA, and an initial volume of 1.6 cubic inches, how would I go about calculating the final temperature and pressure of the steam in the cylinder following adiabatic expansion to a volume of 7 cubic inches? I'm assuming that gamma = 1.4 for this problem. I've attempted to solve this problem using TV^(gamma-1)=Constant, however when I do so I get a final temperature value just above the freezing point of water. I ...
The lesson begins with the slope of an isothermal curve on PV diagram with proper graphical illustrations. Then explanation moves on to slope of adiabatic curves on PV diagram with useful mathematical equations and explanation.
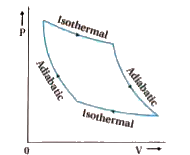
Assume the first stage of the Carnot cycle is an isothermal expansion. During the first stage, all the heat is transferred to work. The second stage is an adiabatic expansion. In the second stage the expansion occurs because the internal energy of the flow is reduced (the temperature is reduced) and work is done on the surroundings. Question 1) Does the first stage continue until the pressure of the fluid is equivalent to the pressure of the gas of the surroundings (i.e. atmospheric)? Or perha...
PV diagrams - part 2: Isothermal, isometric, adiabatic. 4 hours ago So on a PV diagram, an isothermal process is gonna look something like this, it's Draw a p - v diagram for isothermal and adiabatic expansion? 9 hours ago Welcome to Sarthaks eConnect: A unique platform where students can...
PV5/3=constant. We know, mono atomic gas has 3 degree of freedom. > The P-V diagram shows four different possible reversible processes performed on a monatomic ideal gas.Process A is isobaric (constant pressure), process B is isothermal (constant temperature),process C is adiabatic and...
Chapter 19. Kinetic Theory. The Adiabatic Expansion of an Ideal Gas. An adiabatic process is a process for which Q = 0. We can ensure that Q = 0 either by carrying out the process very quickly or by doing it (at any rate) in a well-insulated container.
Can someone help explain to me whats the difference between W=-PV and W = PV, when do I know when to have the negative in front? Also, can someone tell me how I can tell if work is being done by the system vs work being done on the system and the correct sign conventions?? Thanks!
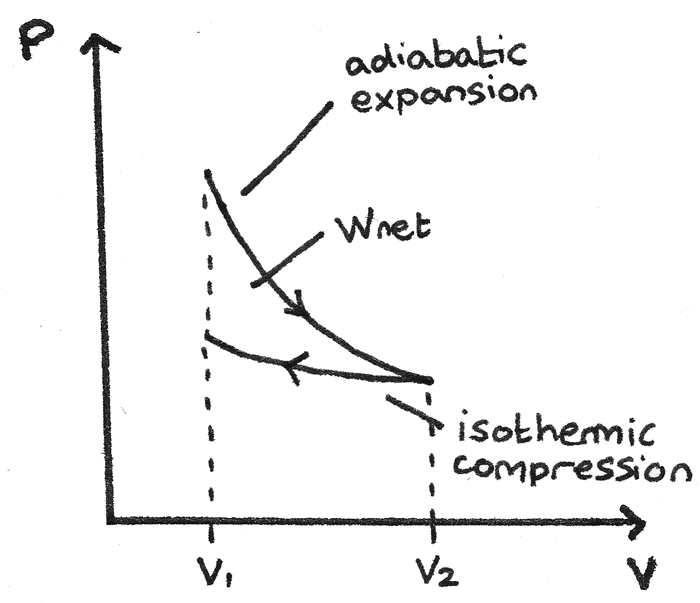
On a pressure–volume (PV) diagram or temperature–entropy diagram, the clockwise and counterclockwise directions indicate power and heat pump cycles, respectively. Relationship to work [ edit ] The net work equals the area inside because it is (a) the Riemann sum of work done on the substance due to expansion, minus (b) the work done to re ...
Learn the concepts of work done during adiabatic expansion including first law of thermodynamics and entropy with the help The adiabatic curve PQ for the process is shown in below figure. The net work done to expand the gas from a volume V1 to Enthalpy of a System The quantity U + PV is known...
Adiabatic expansion against pressure, or a spring, causes a drop in temperature. For an adiabatic free expansion of an ideal gas, the gas is contained in an insulated Some properties of adiabats on a P-V diagram are indicated. These properties may be read from the classical behaviour of ideal gases...
Visit us (http://www.khanacademy.org/science/healthcare-and-medicine) for health and medicine content or (http://www.khanacademy.org/test-prep/mcat) for MCAT related content. These videos do not provide medical advice and are for informational purposes only. The videos are not intended to be a...
Adiabatic expansion of polytropic universe with varying cosmological constant: Models We consider an expanding universe insisting of an adiabatic polytropic uid and a varying cosmological constant. Observational Hubble diagram for Gold data is illustrated in Fig. 1, while the polytropic at and non-at...
The diesel cycle was invented by Rudolph Diesel in 1893. He put forward an idea by which we can attain higher thermal efficiency, with a high compression ratio. All diesel engine works on this cycle. Diesel is used as fuel in this cycle as it can be compressed at higher compression ratio. It is also … Diesel Cycle – Process with P-V and T-S Diagram Read More »
Adiabatic expansion is expansion of a gas without exchange of heat or mass with the surrounding environment. Adiabatic expansion is a situation whereby an external work acts upon a system at the expense of utilizing internal energy of the gas and Can you guess how the T-V diagram looks like?
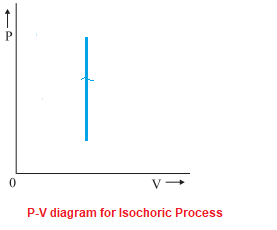
In thermodynamics, an isochoric process, also called a constant-volume process, an isovolumetric process, or an isometric process, is a thermodynamic process during which the volume of the closed system undergoing such a process remains constant.
For a perfect gas, PV = RT or P = RT/V. Substituting P = RT/V in the above equation, we get, As temperature is constant during isothermal expansion and R is a gas constant, = RT (logeV 2-logeV 1) = RT loge (V 2 /V 1) W = 2.3026 RT log10 (V 2 /V 1) According to first law of thermodynamics, heat used up for the expansion of gas = W/J
In the PV diagram of an adiabatic expansion, it is clear that the rate of decrease of pressure is faster than the rate of increase of volume. Why? Since the system is thermally isolated, all the work that is done by the system due to the expansion is all coming from the internal energy of the system. So I get that the internal energy will decrease as the system expands and thus the temperature will decrease also. But temperature is proportional to both pressure and volume. I see no reason as to ...
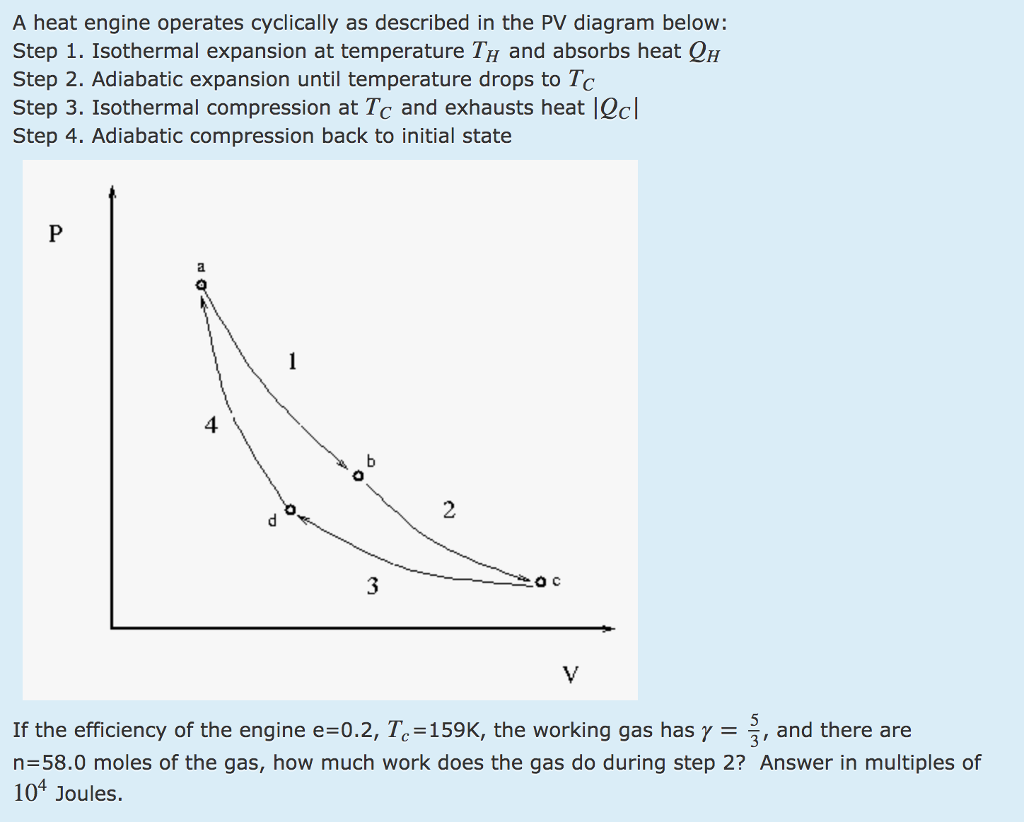
adiabatic expansion, with Th→Tc ; isothermal compression, with ΔQ = - Qc at Tc ; and. If we attempt to compute the entropy change for c→d by finding a point f on the PV diagram such that c→f is adiabatic and f→d is isothermal, we find that we have constructed a Carnot cycle
Adiabatic expansions need not be reversible. Consider this example. I have some moles of a gas in an insulated container .Now the gas adiabatically The work done by the gas is greater than P2(V2-V1) by the amount of this kinetic energy (that area in the PV diagram below the adiabatic gas curve...
Hello, In my exam I had some time ago, there were questions about an ideal gas in a barrel with a moveable piston. The temperature was first raised while maintaining constant volume. Then, the gas expands adiabatically and in this process the temperature drops back to the initial temperature.The question is that it now appears as if the heat absorbed by the gas was subsequently turned into work, which does not comply with the second law of thermodynamics and you have to explain why it actually ...
The example is a carboy filled with n moles of gas that sits at room temperature T1 and volume V1 and pressure P1. Then, the carboy is briefly opened and the gas undergoes an adiabatic expansion against the room pressure, where the gas never leaves the container, so the new values are n, T2, V2, and P2. Then the carboy is closed again, where it is allowed to equilibrate and reach values n, T3, V3, and P3, where T3=T1 and V3 (I think?) = V1. Hopefully that sets the scene. What I really can't wr...
Now, Let's consider the process from 1-2 as Adiabatic Compression & 3-4 as Adiabatic Expansion which was derived below in a hand written format. This is the detailed derivation of Otto Cycle and we can get the efficiency of the cycle by the derivation of Otto cylce.
I was using a spray and it felt ice cold on my skin and when I googled why , I came across Adiabatic effect and or evaporative cooling but I couldn’t really understand. Can somebody explain like I’m 5? Thanks.
An adiabatic process is one in which no heat is gained or lost by the system. This puts a constraint on the heat engine process leading to the adiabatic condition shown below. This condition can be used to derive the expression for the work done during an adiabatic process.
Adiabatic Expansion Efficiency - Free download as PDF File (.pdf), Text File (.txt) or read online for free. Adiabatic Expansion (DQ = 0). Occurs if: • change is made sufficiently quickly • and/or with good thermal isolation. Governing formula: g PV = constant.
Say I have, for example, an air bubble under water. First it has some initial pressure, then it floats upwards quickly so V of the bubble increases while P drops. Now we keep the bubble at some constant height (doesn't matter how we accomplish this task, but we do). How long will it now take for the bubble to be approximately at the pressure of its surroundings again? I have no idea if this is something that would take a microsecond or a whole minute.
In an adiabatic expansion the change in internal energy is negative ie the system does work on the surroundings and the change in internal energy is $\begingroup$ Okay I have one more question. for the adiabatic expansion using the convention opposite to mine , won't the negative signs cancel out...
Adiabatic Process - Work, Heat & Internal Energy, Gamma Ratio, Thermodynamics & Physics PV diagrams - part 2: Isothermal, isometric, adiabatic processes | MCAT | Khan Academy For an adiabatic free expansion of an ideal gas, the gas is contained in an insulated container...
I've studied my thermodynamics textbooks, but I can't find a good example for the expansion of superheated steam in a cylinder where initial pressure, temperature, and volume are known and work, pressure and temperature have to be calculated based off of the final volume. I really appreciate your help!
I need those points for me to manually plot them in a chart in ms word, Googling seems to only provide diagrams in jpeg format. Its even harder to find plot points in Constant P,V lines in TS diagram and constant T,S lines in PV diagram
The operation of the Otto cycle on a p-V diagram is shown in Fig. 5.3. The cycle comprises four processes: adiabatic compression 1 → 2, isochoric heat addition 2 → 3, adiabatic expansion 3 → 4, and isochoric heat removal 4 → 1. Thus, for the Otto cycle, we have V 2 = V 3, V 4 = V 1, CR = V 1 /V 3, and PR = p 3 /p 1.
Can anyone explain why it's drawn like that?
Lesson 42c: PV Diagrams. From the last section, you were probably wondering what happens when we do something like add heat. During an isobaric process the gas is heated and expands from 0.25 m3 to 0.55 m3. Determine the work done by the gas on the cylinder, and sketch a PV diagram of the...


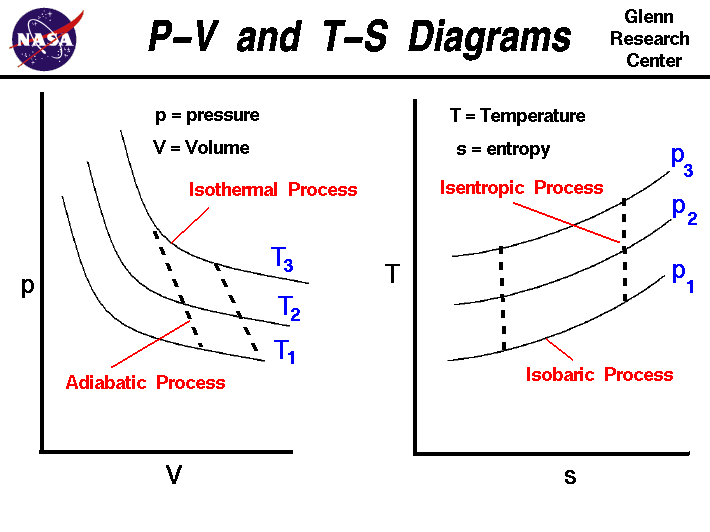



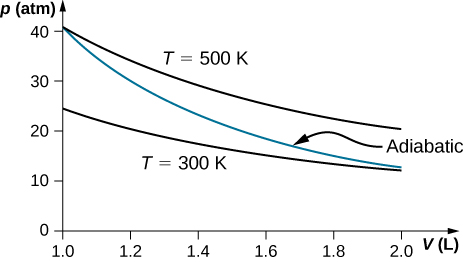

















0 Response to "44 adiabatic expansion pv diagram"
Post a Comment