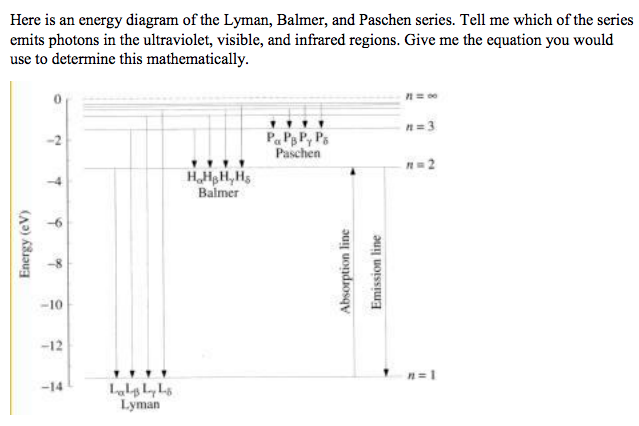
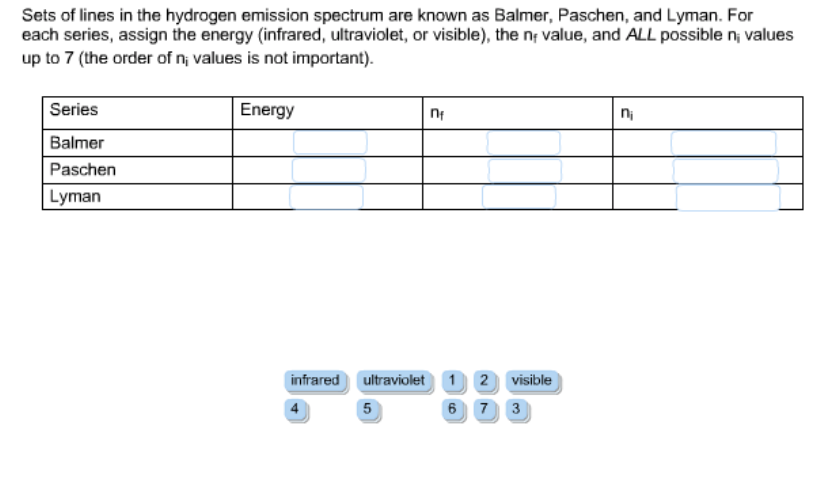
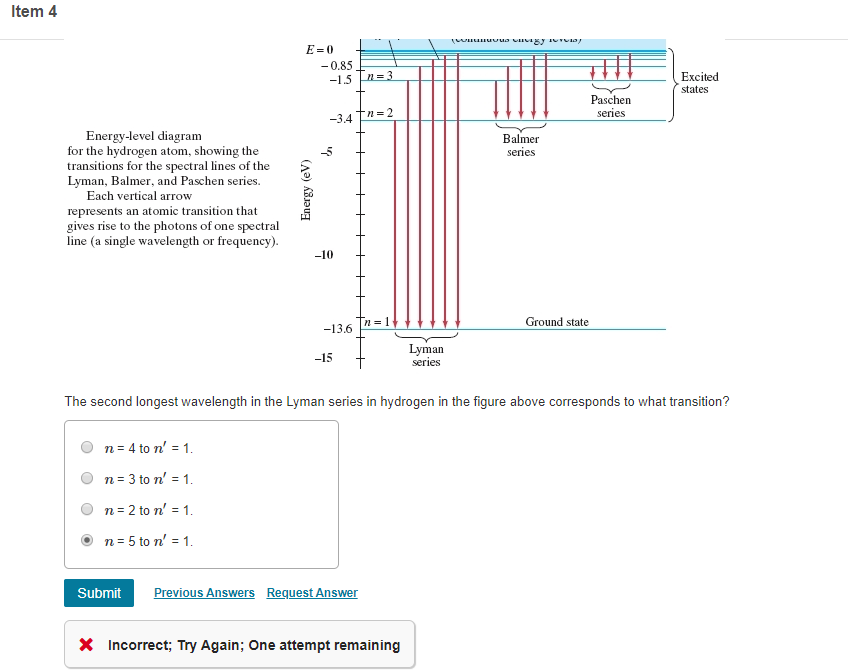
41 choose the correct energy diagram describing the lyman and paschen series.
Exam 2 Flashcards | Quizlet Based on the interactions of light and matter, select all of the correct statements from the following list. Hot solids emit continuous spectra. Bright-line spectra are created by emission. The size of an electron's orbit depends on its energy. Hot gases produce emission spectra. Energy Level and Transition of Electrons | Brilliant Math ... In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to Bohr's theory, electrons of an atom revolve around the nucleus on certain orbits, or electron shells. Each orbit has its specific energy level, which is expressed as a negative value. This is because the electrons on the orbit are "captured ...
PDF ANSWER. Series #2 - LSU Series #2 2. Which series of electron transitions in the energy-level diagram produce the "Balmer" series of lines in a Hydrogen spectrum? ANSWER. Series #2 3. Of the five separate electron transitions that have been labeled with letters in the energy-level diagram, which results in the production (or destruction) of the shortest wavelength ...
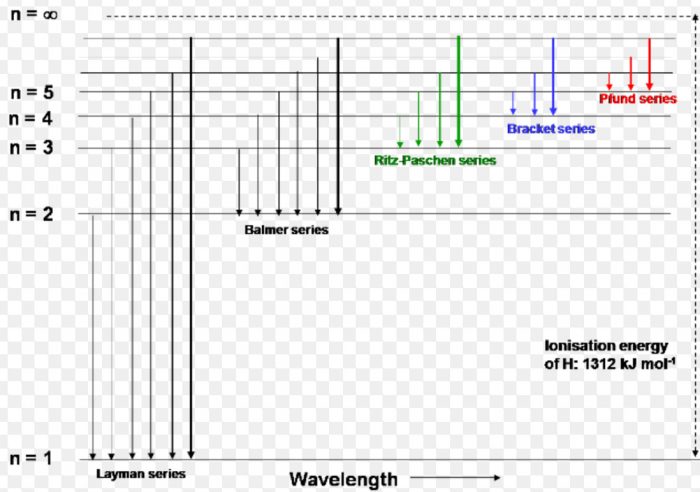
Choose the correct energy diagram describing the lyman and paschen series.
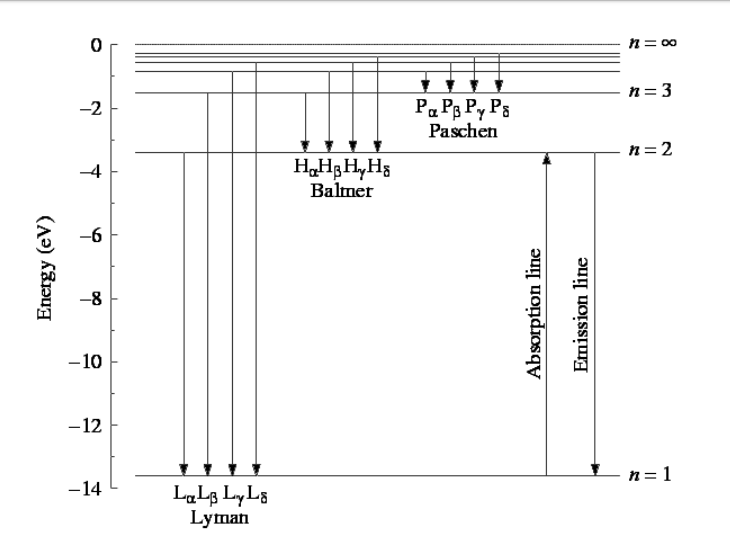
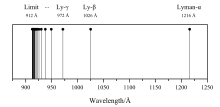
Lyman series - Wikipedia The greater the difference in the principal quantum numbers, the higher the energy of the electromagnetic emission. Contents. 1 History; 2 The Lyman series ... Emission Spectrum of the Hydrogen Atom | Introduction to ... For example, the 2 → 1 line is called Lyman-alpha (Ly-α), while the 7 → 3 line is called Paschen-delta (Pa-δ). Some hydrogen spectral lines fall outside these series, such as the 21 cm line (these correspond to much rarer atomic events such as hyperfine transitions). SK015 Chapter 2 Atomic Structure | Chemistry - Quizizz C. Use the wavelength of the last line in the Lyman series. D. Use the convergence limit of the uv spectrum ... C. Paschen series. D. Brackett series A. Lyman series alternatives B. Balmer series ... The correct set of four quantum numbers for the valence electron of rubidium atom (Z = 37) is ...
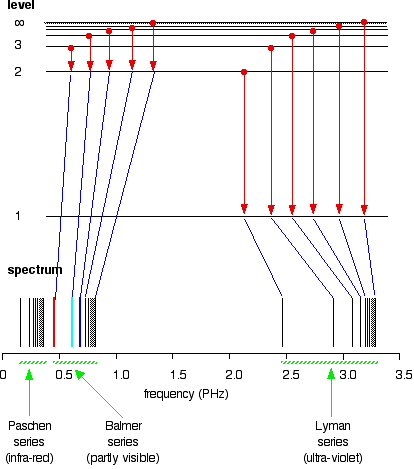
Choose the correct energy diagram describing the lyman and paschen series.. Emission and Absorption Spectra: Bohr, Line Spectrum ... Using this equation, you can calculate the energy of any state. For example, the energy of the stationary state for n=2 will be: E 2 = -2.18×10 -18 J ( 1/2 2)= -0.545×10 -18 J. The energy of an electron is taken as zero when it is not under the influence of the nucleus. In this situation, n=∞ and the atom are called an ionized ... Hydrogen Spectrum: Lyman, Balmer, Paschen, Brackett, and ... We get a Lyman series of the hydrogen atom. It is obtained in the ultraviolet region. This formula gives a wavelength of lines in the Lyman series of the hydrogen spectrum. Different lines of Lyman series are. α line of Lyman series p = 1 and n = 2. α line of Lyman series p = 1 and n = 3. γ line of Lyman series p = 1 and n = 4. atomic hydrogen emission spectrum - chemguide The diagram below shows three of these series, but there are others in the infra-red to the left of the Paschen series shown in the diagram. The diagram is quite complicated, so we will look at it a bit at a time. Look first at the Lyman series on the right of the diagram - this is the most spread out one and easiest to see what is happening. PDF Experiment 7: Spectrum of the Hydrogen Atom PHYS 1493/1494/2699: Exp. 7 - Spectrum of the Hydrogen Atom 2 Introduction The physics behind: The spectrum of light The empirical Balmer series for Hydrogen The Bohr model (a taste of Quantum Mechanics) Brief review of diffraction The experiment: How to use the spectrometer and read the Vernier scale Part 1: Analysis of the Helium (He) spectrum
Answered: What is the energy equivalent of 1… | bartleby A: Potential energy is the product of weight and height of the block from the ground. question_answer Q: MärkxsfH) Problem 1: A mass of 20 grams stretches a spring by 10/169 meters. Stars and GalaxiesDoc added. Must complete the full doc ... Inspect the table above for evidence of such relationships and use these terms to describe the relationships between wavelength, frequency, energy, and velocity. ... The upper right panel labeled "energy level diagram" shows the energy levels ... Lyman, Balmer, and Paschen series. Chemistry Chapter 4 Review Flashcards | Quizlet Lyman series. b.Aufbau series. d. Paschen series. ... How many quantum numbers are needed to describe the energy state of an electron in an atom? a. 1 c. 3 b. 2 d. 4. ... First observed photoelectric effect: shining light of the right frequency level on an element, electrons will jump out of the atoms ... Solved Question 4: Which photon the ground state? (Hint ... Question 6: Complete the energy range values for the 1 excited state G.e. the second orbital) of Hydrogen. Use the simulator to fill out ranges 2 and range 3. The electron can be placed in the 1st orbital by manually dragging the electron or firing a La photon once when the electron is in the ground state. Note also that the electron will ...
6.2 The Bohr Model - Chemistry - opentextbc.ca It is the amount of energy that an electron gains when subjected to a potential of 1 volt; 1 eV = 1.602 × 10 -19 J. Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n = 5 to the orbit with n = 2. Show your calculations. Lab2.rtf - Lesson 2 Lab - The Hydrogen Atom ... - Course Hero Lesson 2 Lab - The Hydrogen Atom Simulator Background Material Carefully read the background pages entitled Energy Levels, Light, and Transitions and answer the following questions to check your understanding. Question 1: (2 points) Complete the following table which compares how the Bohr Model and the Quantum model represent the Hydrogen atom. In some cases they both describe things in the ... Background: Atoms and Light Energy - NASA The ground state of an electron, the energy level it normally occupies, is the state of lowest energy for that electron. There is also a maximum energy that each electron can have and still be part of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and it is considered to be ionized. Emission Spectrum of Hydrogen - Purdue University According to the Bohr model, the wavelength of the light emitted by a hydrogen atom when the electron falls from a high energy (n = 4) orbit into a lower energy (n = 2) orbit.Substituting the appropriate values of R H, n 1, and n 2 into the equation shown above gives the following result.. Solving for the wavelength of this light gives a value of 486.3 nm, which agrees with the experimental ...
(PDF) INSTRUCTOR SOLUTIONS MANUAL | Omar Sosa - Academia.edu Academia.edu is a platform for academics to share research papers.
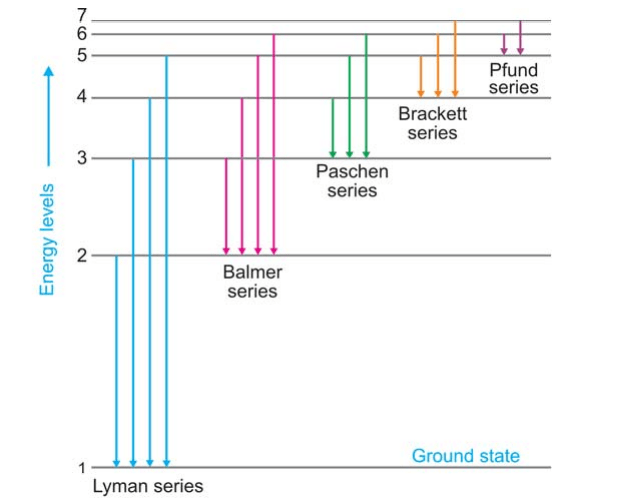
Class 12 - Page 35 - Samacheer Kalvi In each series, the distance of separation between the consecutive wavelengths decreases from higher wavelength to the lower wavelength, and also wavelength in each series approach a limiting value known as the series limit. These series are named as Lyman series, Balmer series, Paschen series, Brackett series, Pfund series, etc.
Bohr Model Questions and Answers - Study.com Sketch and label an energy level diagram for hydrogen, and draw the transitions corresponding to the first four lines in the Balmer series (nf = 2). For the first four lines, indicate which is towa...
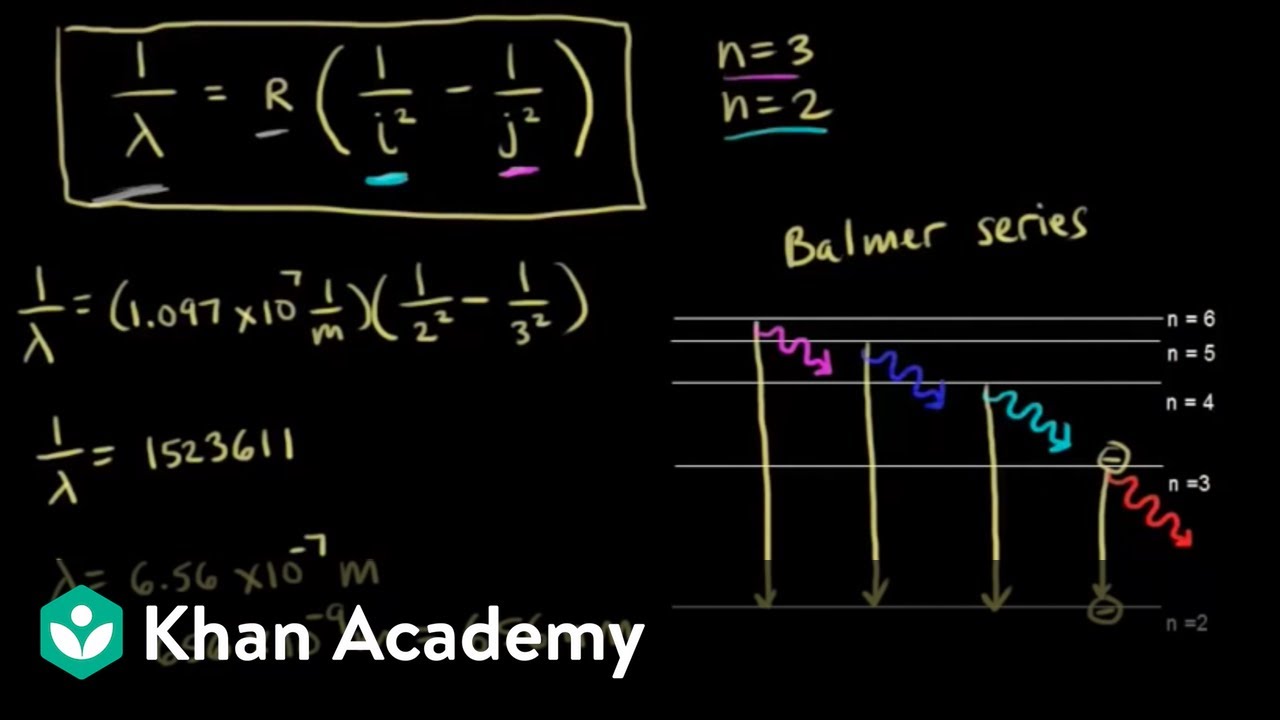
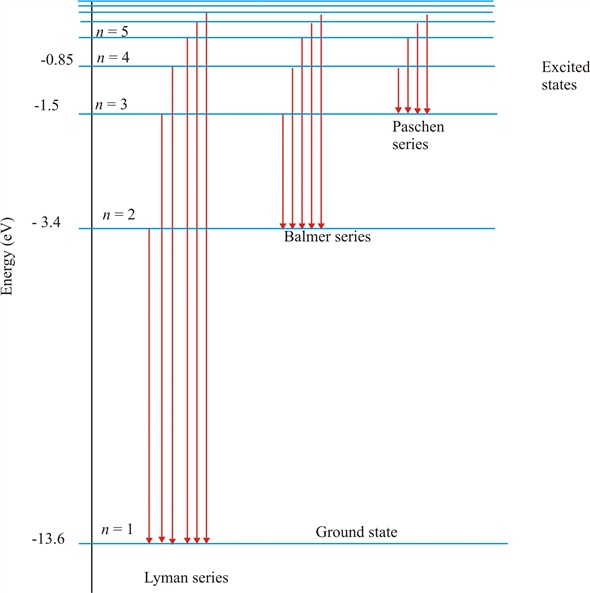
Learn About Quantized Energy Levels | Chegg.com In Lyman series, when electrons jump from higher energy levels to the first energy level n = 1, the frequencies of the emitted photons lie in the Ultraviolet region. In Balmer series, when electrons jump from higher energy levels to the second energy level n = 2, the frequencies of the emitted photons lie in the Visible region.
Balbharati solutions for Physics 12th ... - Shaalaa.com Balbharati solutions for Physics 12th Standard HSC Maharashtra State Board chapter 15 (Structure of Atoms and Nuclei) include all questions with solution and detail explanation. This will clear students doubts about any question and improve application skills while preparing for board exams. The detailed, step-by-step solutions will help you understand the concepts better and clear your ...
Spectral Series- Explained along with Hydrogen spectrum ... Paschen series (n l =3) The series was first observed during the years 1908, by a German physicist Friedrich Paschen. Thus the series is named after him. Paschen series is displayed when electron transition takes place from higher energy states(n h =4,5,6,7,8,…) to n l =3 energy state.
Formation of Spectral Lines | Astronomy - Lumen Learning Figure 2: Energy-Level Diagram for Hydrogen and the Bohr Model for Hydrogen. The right hand side (a) of the figure shows the Bohr model with the Lyman, Balmer, and Paschen series illustrated. A small circle representing the nucleus is enclosed by a larger circle for orbit n = 1, then another larger circle for n = 2 and so on up to n = 5.
Chapter 12 Atoms - NCERT class 12 Physics - for blind and ... Such series were identified in the course of spectroscopic investigations and are known as the Lyman, Balmer, Paschen, Brackett, and Pfund series. The electronic transitions corresponding to these series are shown in Fig. 12.9. FIGURE 12.9 Line spectra originate in transitions between energy levels.
Electron Transitions & Spectral Lines - Video & Lesson ... Transitions like this that occur in the hydrogen atom, the most abundant atom in the universe, can be grouped into well-known series, including the Lyman series, Balmer series, and Paschen series.
Bohr's Model of the Hydrogen Atom - University Physics ... When the series of spectral lines is called the Lyman series.When the series is called the Balmer series, and in this case, the Rydberg formula coincides with the Balmer formula. When the series is called the Paschen series.When the series is called the Brackett series.When the series is called the Pfund series.When we have the Humphreys series.As you may guess, there are infinitely many such ...
Energy, Wavelength and Electron Transitions This transition to the 2nd energy level is now referred to as the "Balmer Series" of electron transitions. Johan Rydberg use Balmers work to derived an equation for all electron transitions in a hydrogen atom. Here is the equation: R= Rydberg Constant 1.0974x10 7 m-1; λ is the wavelength; n is equal to the energy level (initial and final)
Hydrogen spectral series - Wikipedia Lines are named sequentially starting from the longest wavelength/lowest frequency of the series, using Greek letters within each series. For example, the 2 → 1 line is called "Lyman-alpha" (Ly-α), while the 7 → 3 line is called "Paschen-delta" (Pa-δ). Energy level diagram of electrons in hydrogen atom
SK015 Chapter 2 Atomic Structure | Chemistry - Quizizz C. Use the wavelength of the last line in the Lyman series. D. Use the convergence limit of the uv spectrum ... C. Paschen series. D. Brackett series A. Lyman series alternatives B. Balmer series ... The correct set of four quantum numbers for the valence electron of rubidium atom (Z = 37) is ...
Emission Spectrum of the Hydrogen Atom | Introduction to ... For example, the 2 → 1 line is called Lyman-alpha (Ly-α), while the 7 → 3 line is called Paschen-delta (Pa-δ). Some hydrogen spectral lines fall outside these series, such as the 21 cm line (these correspond to much rarer atomic events such as hyperfine transitions).
Lyman series - Wikipedia The greater the difference in the principal quantum numbers, the higher the energy of the electromagnetic emission. Contents. 1 History; 2 The Lyman series ...

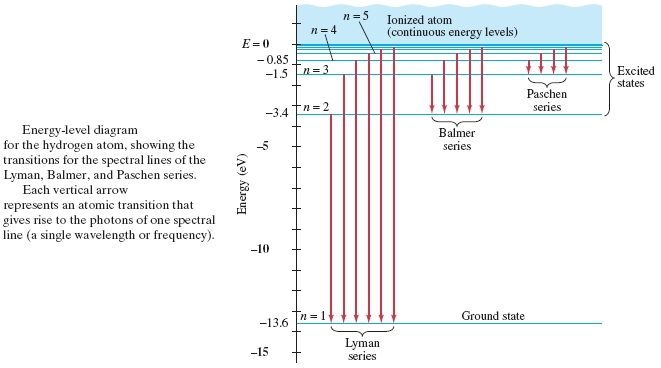

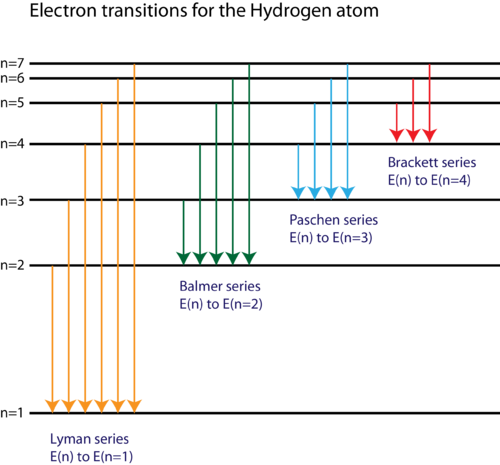

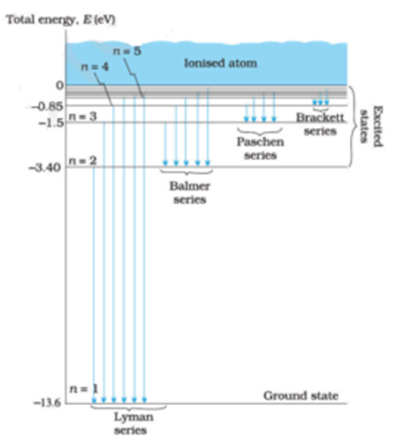

















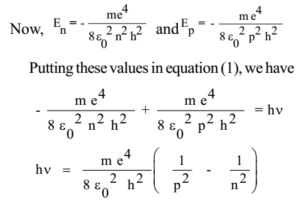

0 Response to "41 choose the correct energy diagram describing the lyman and paschen series."
Post a Comment