41 electron dot diagram for iodine
Iodine difluoride (IF2-) lewis structure, molecular ... For iodine atom ⇒ Valence electrons of iodine = 7 ⇒ Nonbonding electrons on iodine = 6 ⇒ Bonding electrons around iodine (2 single bonds) = 4 ∴ (7 - 6 - 4/2) = -1 formal charge on the iodine central atom. ∴ The overall formal charge in IF2- lewis structure is -1 which is equal to the charge on the ion (IF2- molecule has one negative charged ion). Hydrogen Iodide (HI) Lewis Structure HI is a very easy lewis structure to draw due to its simplicity. HI lewis structure. There are only one hydrogen atom and one iodine atom in HI molecule. In the lewis structure of HI, hydrogen atom has made a single bond with iodine atom. Steps of drawing lewis structure of HI. When we draw a lewis structure, there are several steps to follow.
Electron Dot Diagrams and Lewis Structures Flashcards ... electron dot diagram for Iodine. electron dot diagram for Boron. ... Lewis structure for ONCl. 4 dots around N, double bonded to O, 2 dots around O, single bonded to Cl, 6 dots around Cl. Related questions. QUESTION. T/F: a solution with a non-volatile solute has a higher vapor pressure than a pure solvent of the solution.

Electron dot diagram for iodine
How do you draw an electron dot diagram for iodine? - Answers There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for ... Iodine Electron Dot Diagram - schematron.org iodine has 7 valence electrons. So you draw seven dots, 2 on each side of the letter I. and on one side you just put one dot, I think its best to put. The left diagram shows a Lewis dot structure of sodium with. ASK A BRAND Likewise, they can be used .. The left shows an iodine atom with one lone pair. IO3- Lewis Structure - How to draw the Electron Dot ... Drawing the Lewis Structure for IO 3- (Iodate Ion) With IO 3- be sure to add an additional valence electron to your total because of the negative sign. There are a total of 26 valence electrons in IO 3-. Iodine is in Period 5 on the Periodic table. Therefore it can hold more than 8 valence electrons.
Electron dot diagram for iodine. topblogtenz.com › nitrogen-triiodide-ni3-lewisNitrogen triiodide (NI3) lewis dot structure, molecular ... The molecular geometry of NI3 is trigonal pyramidal, and electron geometry is tetrahedral because the lone pair present on the central atom creates repulsion between adjacent bonded pairs of electrons, as a result, two iodine atoms in equatorial position pushes far apart giving its molecular geometry the same as a trigonal pyramid. How to Draw the Lewis Dot Structure for I: Iodine - YouTube A step-by-step explanation of how to draw the I Lewis Dot Structure.For the I structure use the periodic table to find the total number of valence electrons ... › embedWelcome to CK-12 Foundation | CK-12 Foundation FlexBook® Platform, FlexBook®, FlexLet® and FlexCard™ are registered trademarks of CK-12 Foundation. Iodine (I2) Molecule Lewis Structure Iodine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of iodine atoms. valence electrons given by iodine atoms = 7 * 2 = 14 Total valence electrons = 14 Total valence electrons pairs
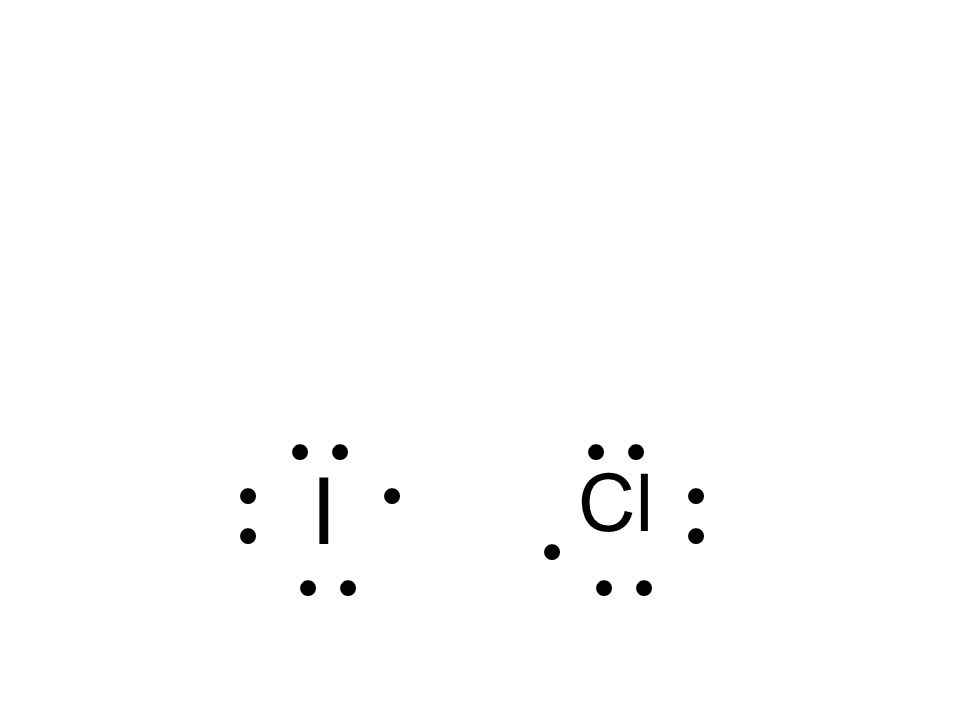
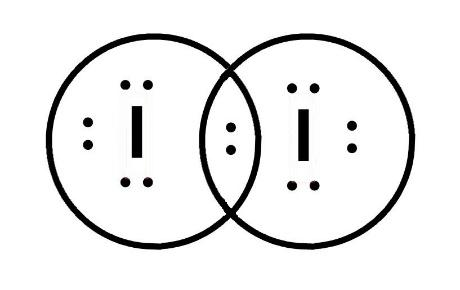
chem.libretexts.org › Courses › Furman_University4.8: Covalent Bonding and Formula Writing - Chemistry LibreTexts Jan 23, 2021 · Draw the Lewis diagram for each compound. a molecule composed of two chlorine atoms; a molecule composed of a hydrogen atom and a bromine atom; Solution. Chlorine has the same valence shell electron configuration as fluorine, so the Lewis diagram for a molecule composed of two chlorine atoms is similar to the one for fluorine: How to draw I2 Lewis structure - Science Education and ... Key Points To Consider When Drawing The I2 Electron Dot Structure. A three-step approach for drawing the I2 Lewis structure can be used. The first step is to sketch the Lewis structure of the I2 molecule, to add valence electrons around the two iodine atoms, and the final step is to combine the two iodine diatomic atoms to get the I2 Lewis Structure. techiescientist.com › o3-lewis-structureO3 Lewis Structure, Molecular Geometry ... - Techiescientist 1 day ago · It uses quantum mechanics to give us a detailed almost explanatory diagram of the bonding nature inside a molecule. Here is a diagrammatic representation of the MO diagram of ozone. Ozone is a trigonal planar molecule. Hence, as we take one p orbital from each atom of oxygen(O3), we focus on the 4 electron H3- anion. techiescientist.com › i3-lewis-structureI3 Lewis Structure, Molecular Geometry, Hybridization ... Mar 18, 2022 · We also have an additional electron to provide the overall negative charge to the I3 molecule. Total number of valence electrons = 3*7+ 1 =21 + 1 =22. 2. Since all the atoms are iodine, one of these plays the role of the central atom. 3. As you can see in the above diagram, the skeletal structure of I3 with the negative charge has been drawn. 4.
I2 Lewis Structure - How to Draw the Dot Structure for I2 ... A step-by-step explanation of how to draw the I2 Lewis Dot Structure (Iodine Gas).For the I2 structure use the periodic table to find the total number of val... quizlet.com › 414314226 › atomic-numbers-andAtomic numbers and electron configurations assignment and ... Dot structures make it easy to count electrons and they show the number of electrons in each electron shell. Arrow and line diagrams show the spin of electrons and show every orbital. Written configurations require minimal space and show the distribution of electrons between subshells. What should the electron dot diagram for iodine look like ... What should the electron dot diagram for iodine look like? Chemistry Covalent Bonds Drawing Lewis Structures. 1 Answer Jahan Psyche Oct 24, 2015 Answer link. Related questions. What are lewis dot structures used for? What is the lewis structure for #SO_2#? How do you draw the lewis structure for ions? ... › topics › chemistryPhotoluminescence Spectroscopy - an overview | ScienceDirect ... The excited electron in the conduction band relaxes quickly, typically within 15–25 ps [61], to the band edge via inter- or intra-sub-band scattering and then recombines with the hole either radiatively by emitting a photon or non-radiatively by passing the energy on to one or more phonons, to ‘trapping’ states that are created by ...
PDF Lewis electron dot diagram for potassium iodide Concentrated solution of potassium iodide and. Ioduro / iodine by Lewis Ford Lewis Structure - Wikipedia, free encyclopedialewis structures (also known as Lewis Dot diagrams, electronic point diagrams. Elements of groups from 15 to 18, such as phosphorus, sulphur, iodine and xenon. Lewis Structures for
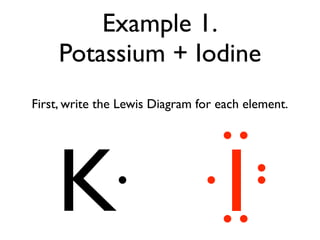
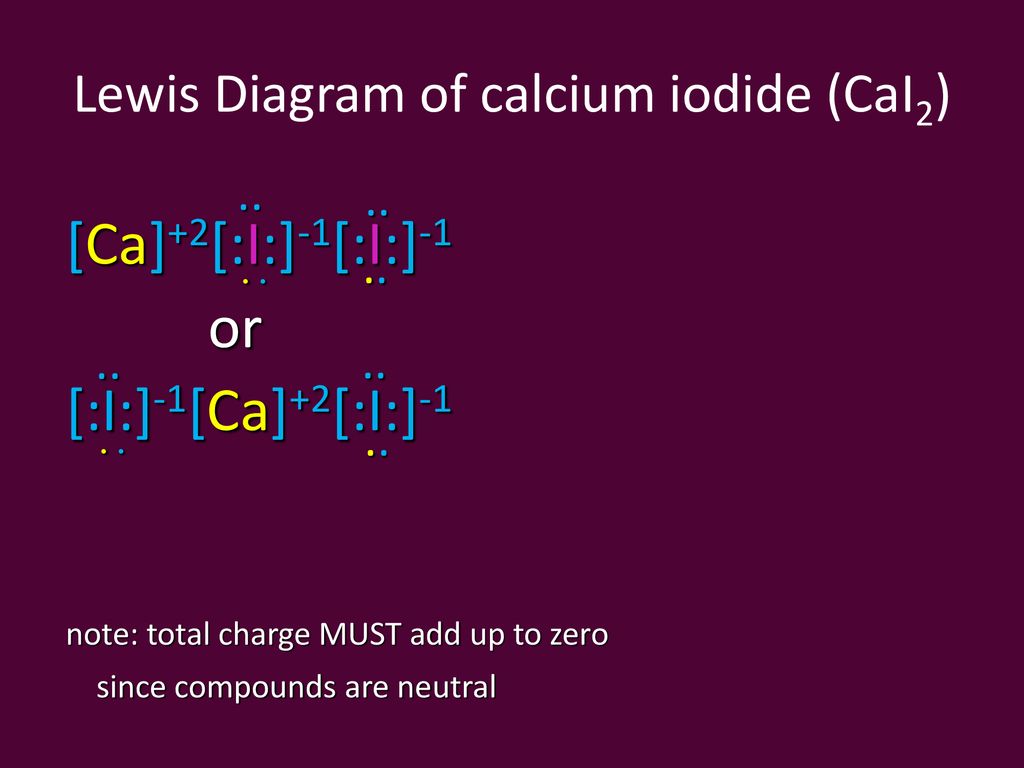
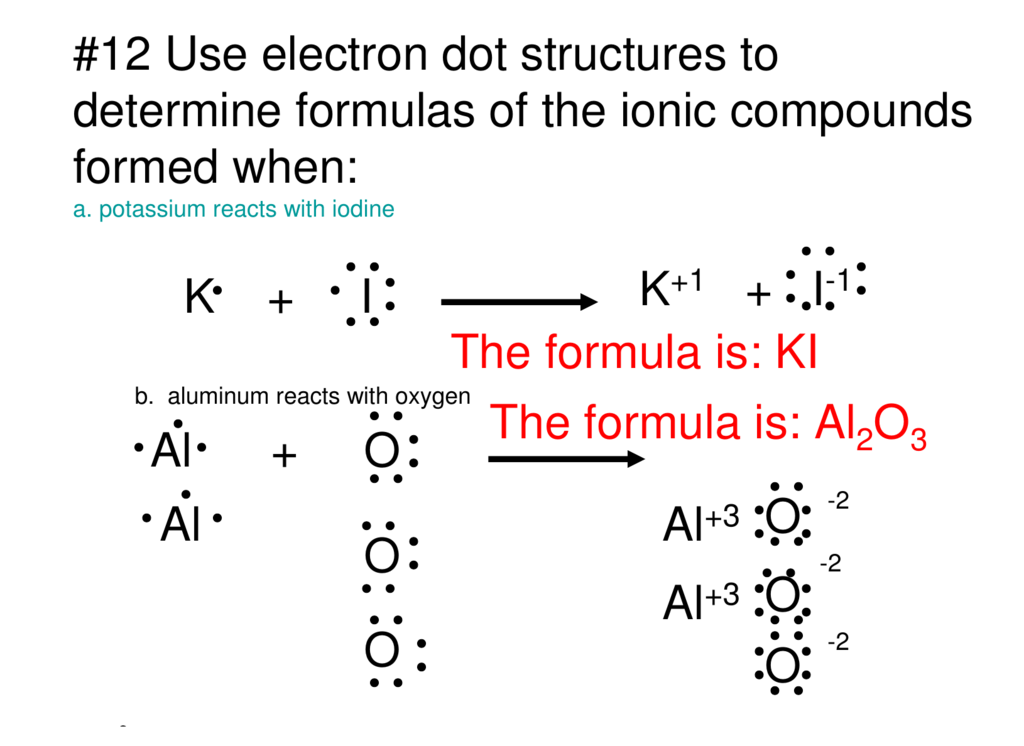
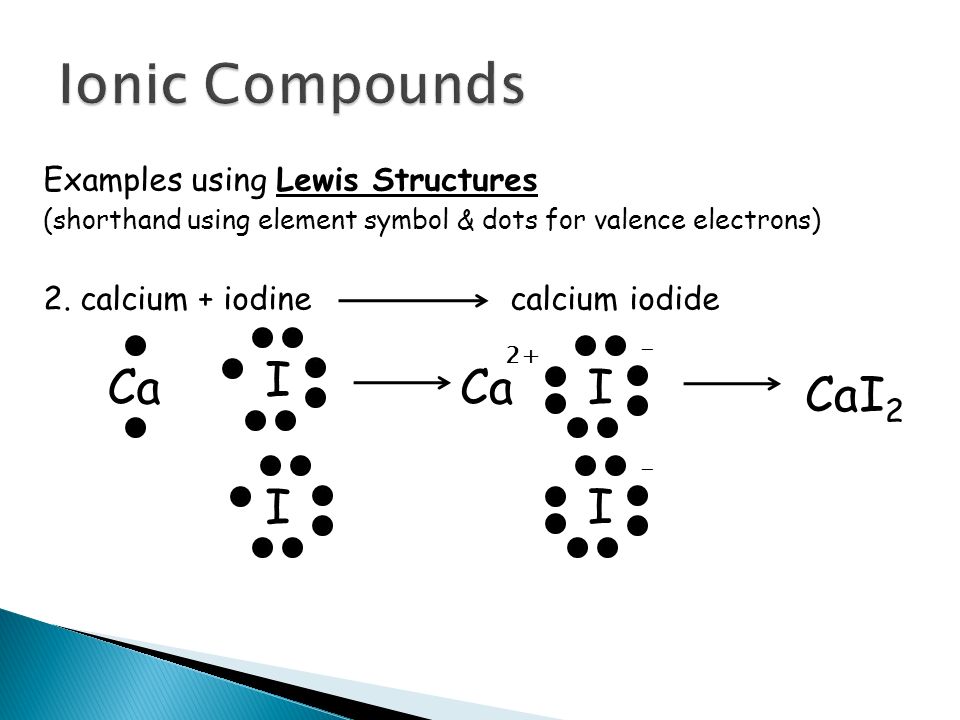
PPTX Topic: Lewis Dot Diagrams for Ionic Compounds NaCl's Lewis structure: ... What is the ionic compound formed from calcium and iodine? Calcium - metal - 2 valence electrons - loses both electrons [Ca]+2. Iodine - nonmetal - 7 valence electrons - gains 1 electron ... Lewis Diagram of Calcium Iodide ...
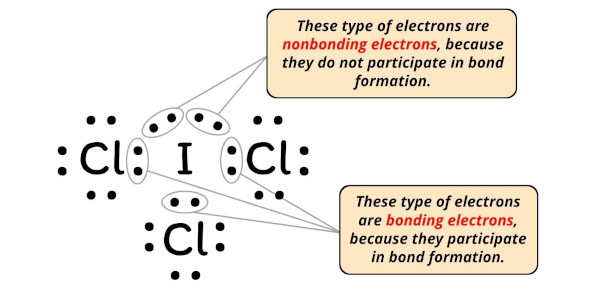
ICl5 Lewis Structure, Geometry, Hybridization, and ... The electronic configuration of iodine is [Kr]4d105s25p5. As we can see, there are seven electrons in the valence shell of iodine. Secondly, the electronic configuration of chlorine is 1s22s22p63s23p5. Since chlorine and iodine belong to the same group i.e. group 17, there are seven electrons in the valence shell of chlorine as well.
I3- Lewis Structure, Shape, Hybridization and Polarity Also, iodine is in the seventh group of the periodic table and has seven valence electrons in its outer orbit. We have three molecules of iodine here which along with an extra electron which gives it a negative charge. So the total number of valence electrons are : 7×3 + 1= 22. There are 22 valence electrons in total in this molecule.
What is the Lewis structure for iodine? What is the Lewis structure for iodine? It has three singly bonded f atoms with two pair of dots on the i atom. Iodine is a halogen with seven valence electrons so you would draw seven dots which two dots on three sides of the iodine molecule and one on one side surrounded by a bracket with a negative sign on the top right corner.
Iodine Lewis Dot Diagram - schematron.org The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the . Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model .
Lewis Structure for ICl4- - UMD Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons. Also note that you should put the ICl4- Lewis structure in brackets with as 1- on the outside to show that it is an ion with a negative one charge.
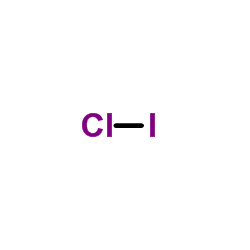
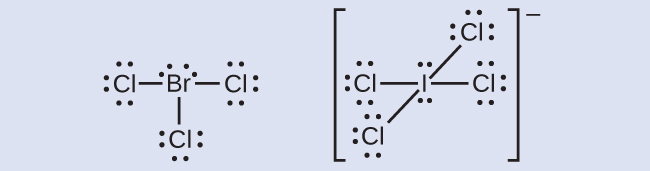
Iodine Electron Dot Diagram - wiringall.com For Iodine we have 7 valence electrons, and 7 for the Chlorine; total of 14 valence electrons for the ICl Lewis structure. We'll put the Iodine here, and the Chlorine right next to it. iodine w/ six electrons around it, bromine w/ six electrons around it and two electrons between them. that will maintain an octet around both atoms.
ICl3 Lewis Structure, Molecular Geometry, Hybridization ... The ground state electronic configuration of Iodine is [Kr] 4d105s25p5. There is only one unpaired electron but we need three unpaired electrons for the formation of three bonds with three chlorine atoms. Hence, one of the electrons from the 5p orbital will promote to 5d orbital for the formation of three bond pairs with three chlorine atoms.
ICl2- lewis structure, molecular geometry, bond angle ... ICl2- lewis structure contains one iodine atom at the middle position whereas two chlorine atoms at the surrounding position. There are three lone pairs present on the central atom of ICl2- lewis structure. Also, the iodine central atom in ICl2- lewis structure violates the octet as it is holding more than 8 electrons in its octet shell.
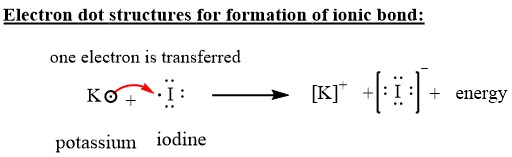
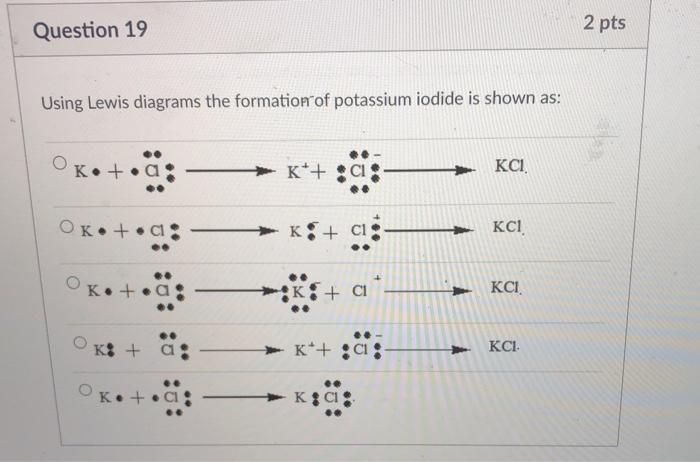
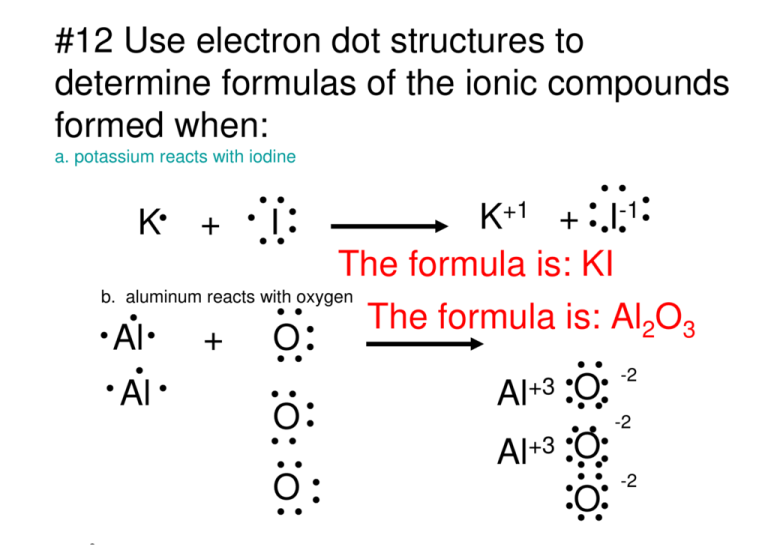
How to draw KI Lewis Structure? - Science Education and ... To sketch the KI Lewis structure by following these instructions: Step-1: KI Lewis dot Structure by counting valence electrons on the iodine atom. Step-2: Lewis Structure of KI for counting valence electrons around the terminal potassium atoms. Step-3: Lewis dot Structure for KI generated from step-1 and step-2.
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
What should the electron dot diagram for iodine look class ... Hint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, if they exist in the molecule. Iodine is the molecule consisting of two atoms of iodine bonded together satisfying both the planes. Complete answer: Let us study the concept;
ICl Lewis Structure - How to draw the Electron Dot ... For Iodine we have 7 valence electrons, and 7 for the Chlorine; total of 14 valence electrons for the ICl Lewis structure. We'll put the Iodine here, and the Chlorine right next to it. We have a total of 14 valence electrons. We'll put 2 between atoms to form the chemical bond, and we'll go around the outside.
Lewis dot structure for Bromine and Iodine? - Answers Iodine fluoride has the molecular formula of IF3. It is composed of one iodine (I) and three fluoride (F) atoms. The Lewis dot structure for iodine fluoride is (the four eletrons above iodine ...
Lewis Structure for IF5 - UMD Drawing the Lewis Structure for IF 5. Video: Drawing the Lewis Structure for IF 5. Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). In the Lewis structure for IF5 you'll need to put a total of 12 valence electrons on the Iodine atom in order to draw the Lewis structure.
Lewis Dot Diagram Iodine Lewis Dot Diagram Iodine Comprehensive information for the element Iodine - I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . The left diagram shows a Lewis dot structure of sodium with. Likewise, they can be used .. The left shows an iodine atom with one lone pair. When we write the .
What is the ionic bond for magnesium and iodine? To draw the Lewis electron dot diagram we picture in our minds the symbol for Mg in a box with all of its core electrons (i.e., 1s 2 2s 2 2p 6). Then we place the valence electrons around the sides of the box with each side representing an orbital in the outermost energy level.
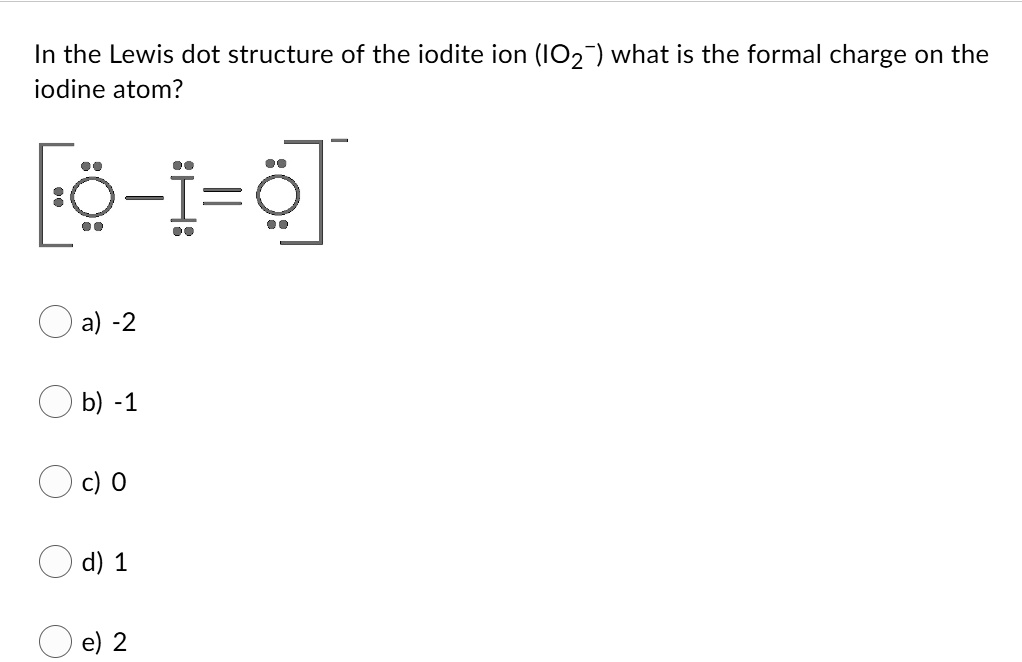
IO3- Lewis Structure - How to draw the Electron Dot ... Drawing the Lewis Structure for IO 3- (Iodate Ion) With IO 3- be sure to add an additional valence electron to your total because of the negative sign. There are a total of 26 valence electrons in IO 3-. Iodine is in Period 5 on the Periodic table. Therefore it can hold more than 8 valence electrons.
Iodine Electron Dot Diagram - schematron.org iodine has 7 valence electrons. So you draw seven dots, 2 on each side of the letter I. and on one side you just put one dot, I think its best to put. The left diagram shows a Lewis dot structure of sodium with. ASK A BRAND Likewise, they can be used .. The left shows an iodine atom with one lone pair.
How do you draw an electron dot diagram for iodine? - Answers There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram. To make the electron dot diagram you put the electron symbol and put a dot on one of the sides for ...
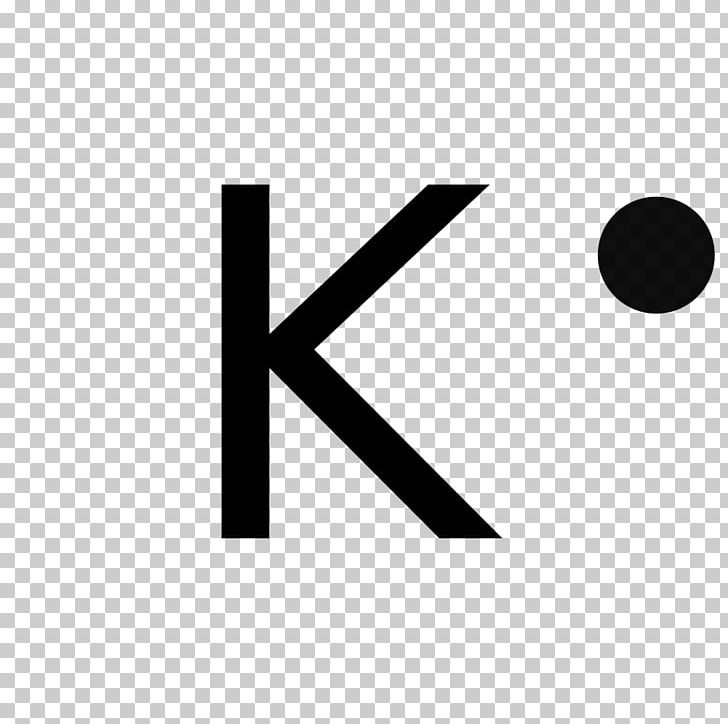



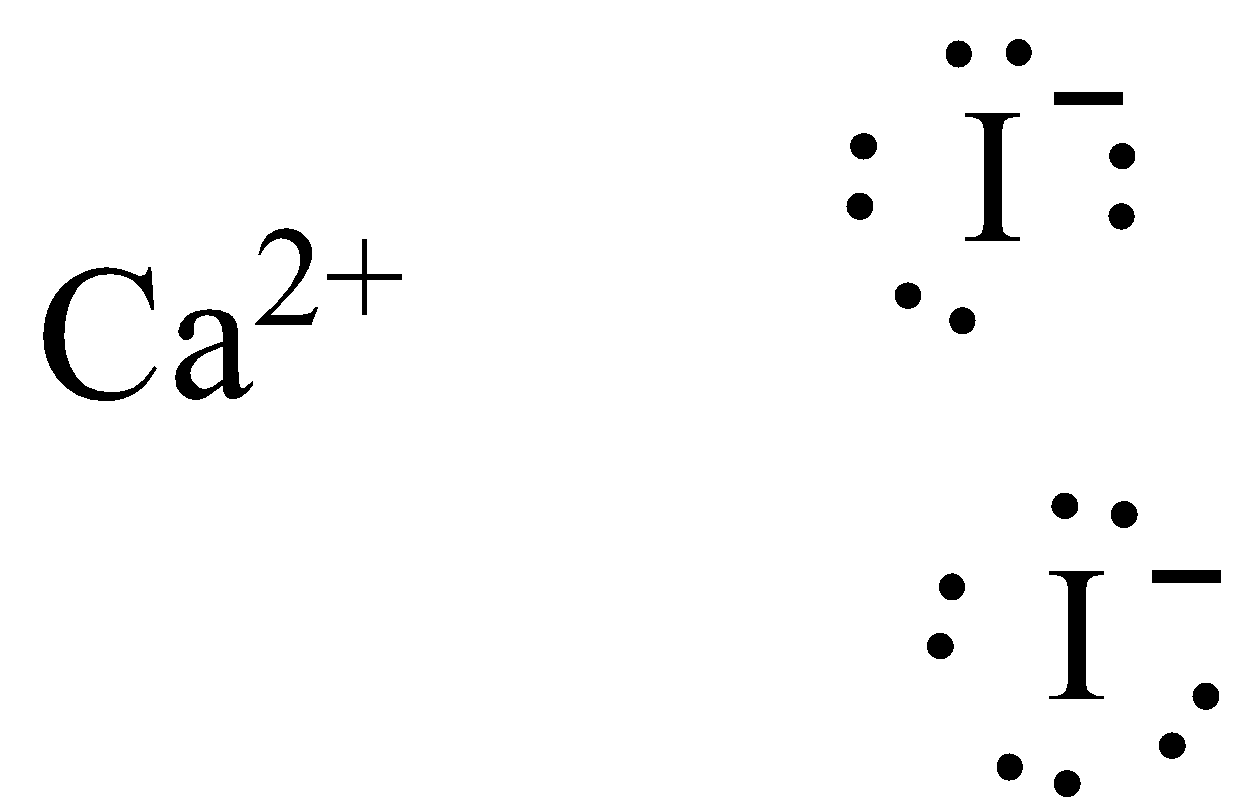












![Expert Verified] Which Lewis dot structure represents bonding ...](https://us-static.z-dn.net/files/dcb/eb0cb0a538e5db52ff692f01fa6f54ea.png)




0 Response to "41 electron dot diagram for iodine"
Post a Comment