41 selenium electron dot diagram
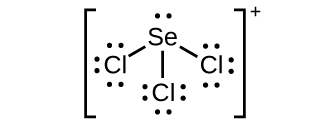
How to Draw the Lewis Dot Structure for SeBr4: Selenium ... A step-by-step explanation of how to draw the SeBr4 Lewis Dot Structure.For the SeBr4 structure use the periodic table to find the total number of valence el... Selenium | Se - PubChem Selenium | Se | CID 6326970 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards ...
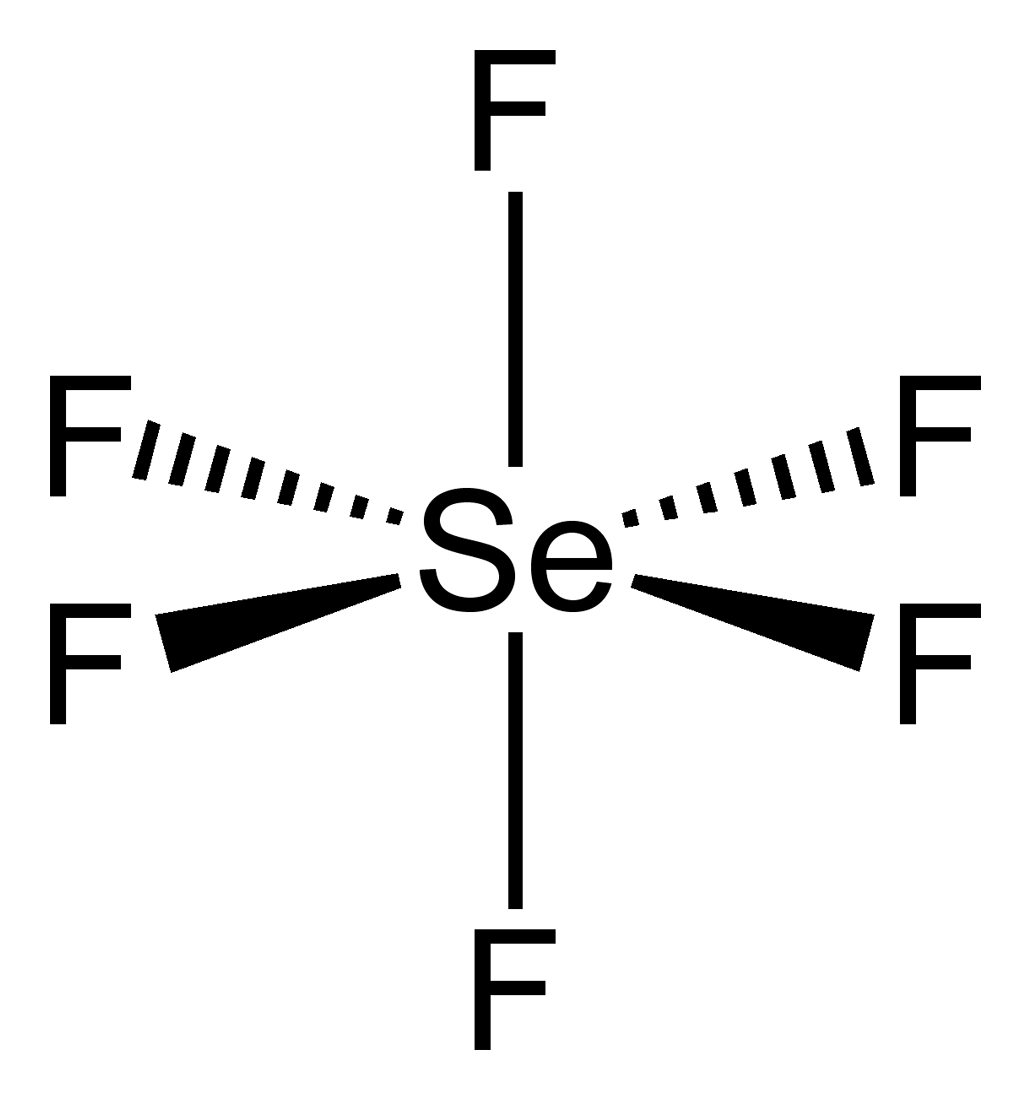
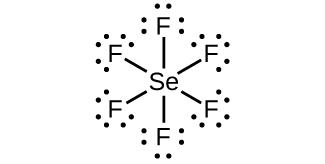
SeF6 Lewis Structure, Geometry, Hybridization, and ... Step 2: Draw the lewis dot structure for elements. We draw the Lewis structure of elements by arranging the valence shell electrons around the element's chemical symbol. The chemical symbols for Selenium and fluorine are Se and F, respectively. The Lewis dot structure for Se and F are as follows-
Selenium electron dot diagram
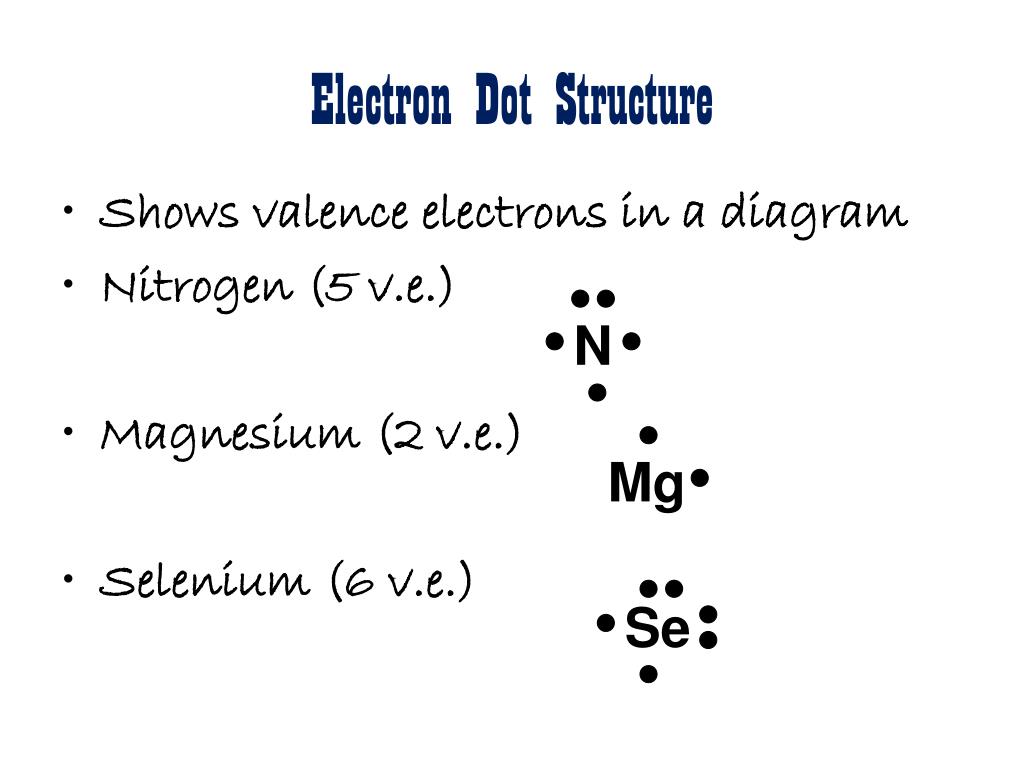
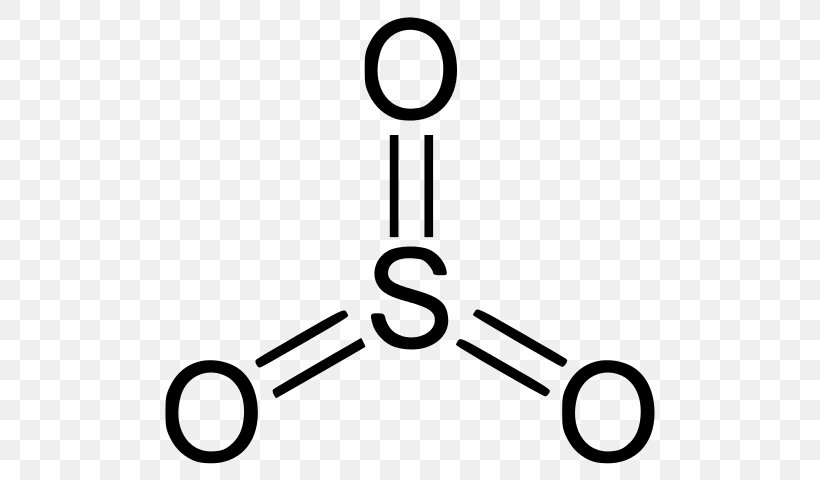
Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3 s2 3 p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: The valence electron configuration for selenium is 4 s2 4 p4. Lewis Diagram For Seo3 Lewis Diagram For Seo3. A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. The Os. Draw all possible resonance structures for the molecule selenium trioxide (SeO3) . The structure and arrows are given, simply add the remaining bonds and lone. (1 ... Draw the Lewis dot structure for SeO3 and answer the ... Draw the Lewis dot structure for {eq}SeO_3 {/eq} and answer the following questions. ... with each electron domain being between an oxygen atom and the central selenium atom. b. The electron ...
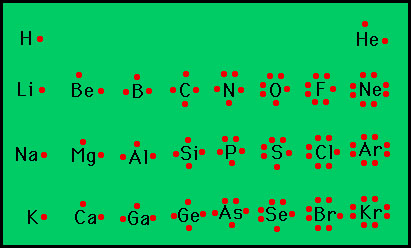
Selenium electron dot diagram. Selenium Orbital Diagram - schematron.org By looking at the electron configuration of selenium, it is possible to determine how many electrons are in each sub-shell. Molecular orbital diagram There are five sub-shells, but only four of them are used by naturally occurring elements: s, p, d and f. Each sub-shell accommodates a certain number of electrons. Whats the electron dot structure for selenium? - Answers What is the electron dot structure for selenium? Because selenium is in the 16th column on the periodic chart, it has 6 valence electrons. Thus, the Lewis structure (or electron dot structure) for ... How to draw H2Se Lewis Structure? - Science Education and ... Step-1: H2Se Lewis dot Structure by counting valence electrons on the selenium atom To calculate the valence electron of each atom in H2Se, look for its periodic group from the periodic table. The oxygen and hydrogen group families, which are the 16th and 1st groups in the periodic table, are both made up of selenium and hydrogen atoms ... 3A2 Lewis Dot Structures - Advanced Chemistry A Lewis dot structure for an element shows two things: the symbol with an appropriate number of dots representing the proper number of valence electrons. Examples: The standard way of dot placement in a Lewis dot structure has an electron placed in the four directions (North, South, East and West), and then when more than 4 are needed listed ...
5.3 Electron Configuration Flashcards - Quizlet This element exists in the solid state at room temperature and at normal atmospheric pressure and is found in emerald gemstones. It is known to be one of the following elements: carbon, germanium, sulfur, cesium, beryllium, or argon. Identify the element based on the electron-dot structure. a) Si 1s²2s²2p⁶3s²3p². What is the Lewis dot structure for SSE2? - Quora Answer: I'm just guessing; but I'm almost sure you meant SeS2 that is selenium disulfide, not SSe2 that is sulfur diselenide. That is because "S" is a little more electronegative than "Se" and tends to work with a "-2" -non real- oxidation state, but it is true that are almost equal in electrone... How Many Valence Electrons Does Selenium Have ... On the periodic table selenium is element number 34. It is located in group 16 below oxygen and sulfur. The electrons in the 4s and 4p shells combined are the valence electrons meaning there are 6 valence electrons on. Valence electrons are characteristics of metals. It accepts electrons rather than releases them. A 2 b 4 c 6 d 8 8. How to Draw the Lewis Dot Structure for SeF2: Selenium ... A step-by-step explanation of how to draw the SeF2 Lewis Dot Structure (Selenium difluoride).For the SeF2 structure use the periodic table to find the total ...
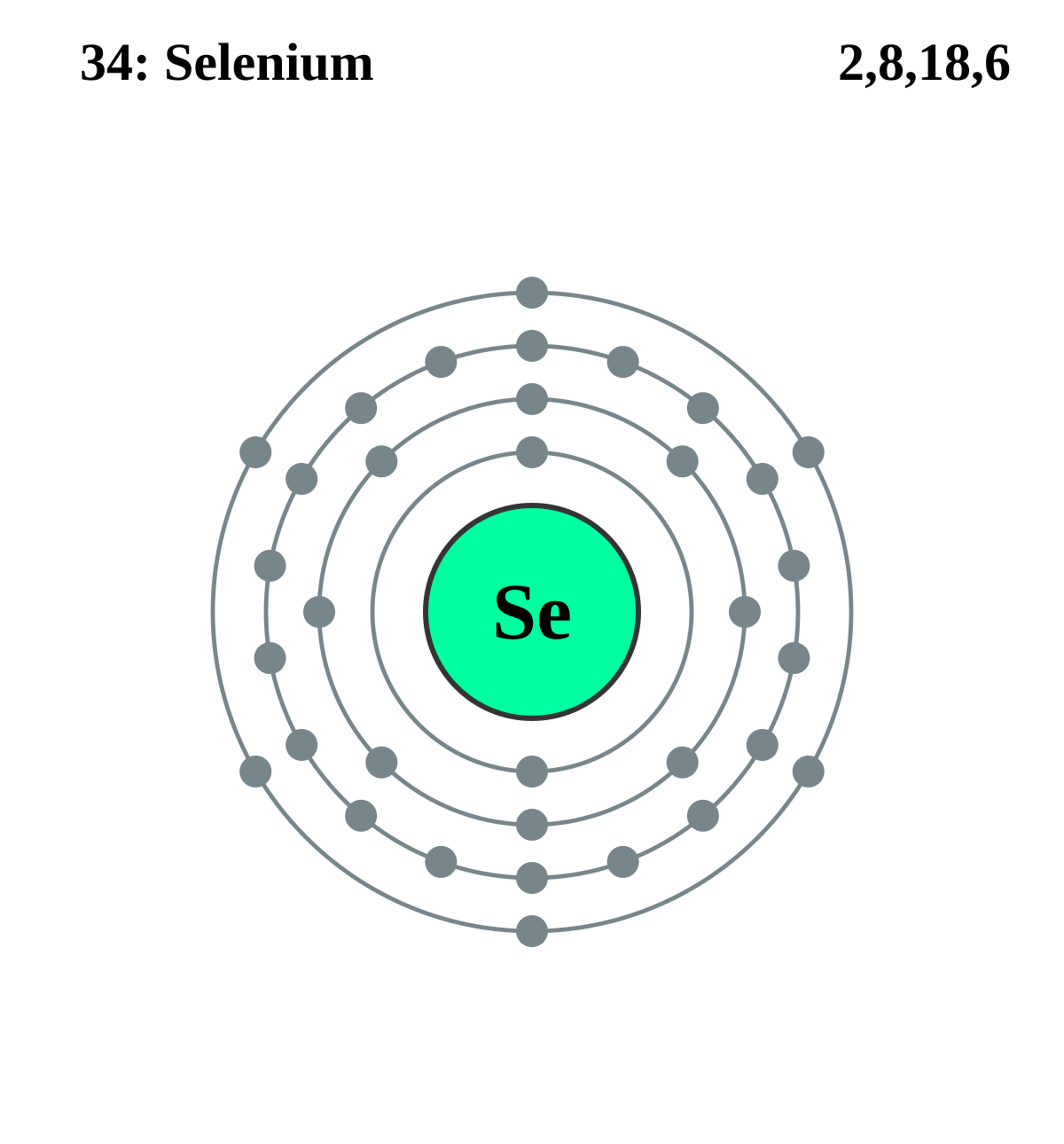
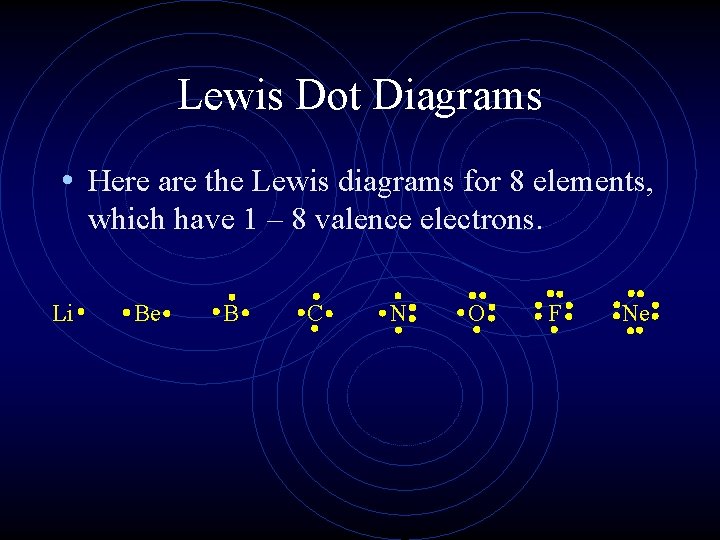
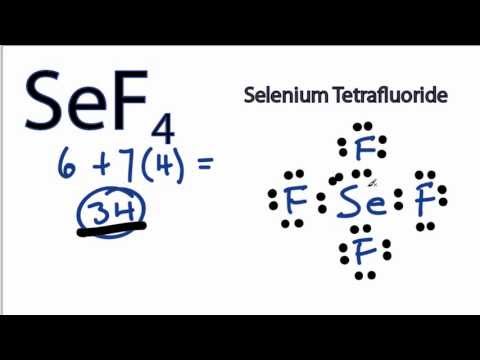
Lewis Electron Dot Diagrams - GitHub Pages (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. ... selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1. SeCl2 Lewis Structure (Selenium Dichloride) - YouTube SeCl2 is a chemical formula for Selenium Dichloride. It comprises one Selenium and two Chlorine atoms. Here in this video, we will help you determine the Lew... Selenium tetrafluoride (SeF4) lewis dot structure ... Selenium tetrafluoride (SeF4) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Selenium tetrafluoride is an inorganic compound that appears as a colorless liquid having the chemical formula SeF4. It can react with water and forms hydrofluoric acid and selenous acid. Selenium in the SeF4 molecule has a +4 oxidation state. What Is the Electron Configuration of Selenium? The electron configuration of an atom shows how the electrons are arranged in the atom's energy levels. This configuration conveys a lot of important information about an element. By looking at the electron configuration of selenium, it is possible to determine how many electrons are in each sub-shell. There are five sub-shells, but only four ...
What is the noble gas electron configuration for selenium ... What is the ground state electron configuration for Na+? Examples: Na has a ground-state electronic configuration of 1s2 2s2 2p6 3s1. Removing the 3s electron leaves us with the noble gas configuration 1s2 2s2 2p6, so a sodium ion is Na+. What is the ground state electron configuration for the ni3+ cation? It is [Ar]3d84s2 .
Periodic Table of Elements: Selenium - Se ... Comprehensive information for the element Selenium - Se is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
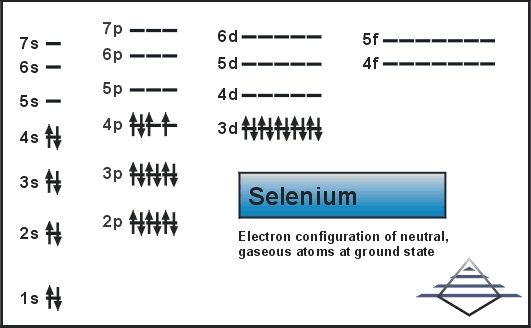
Orbital Diagram For Selenium - schematron.org In writing an. Answer to orbital diagram for selenium home / study / science / chemistry / chemistry questions and answers / Orbital Diagram For Selenium. here is the electronic configuration. Z=34 so 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4.Selenium (Se) has an atomic mass of Find out about its chemical and physical properties, states, energy ...
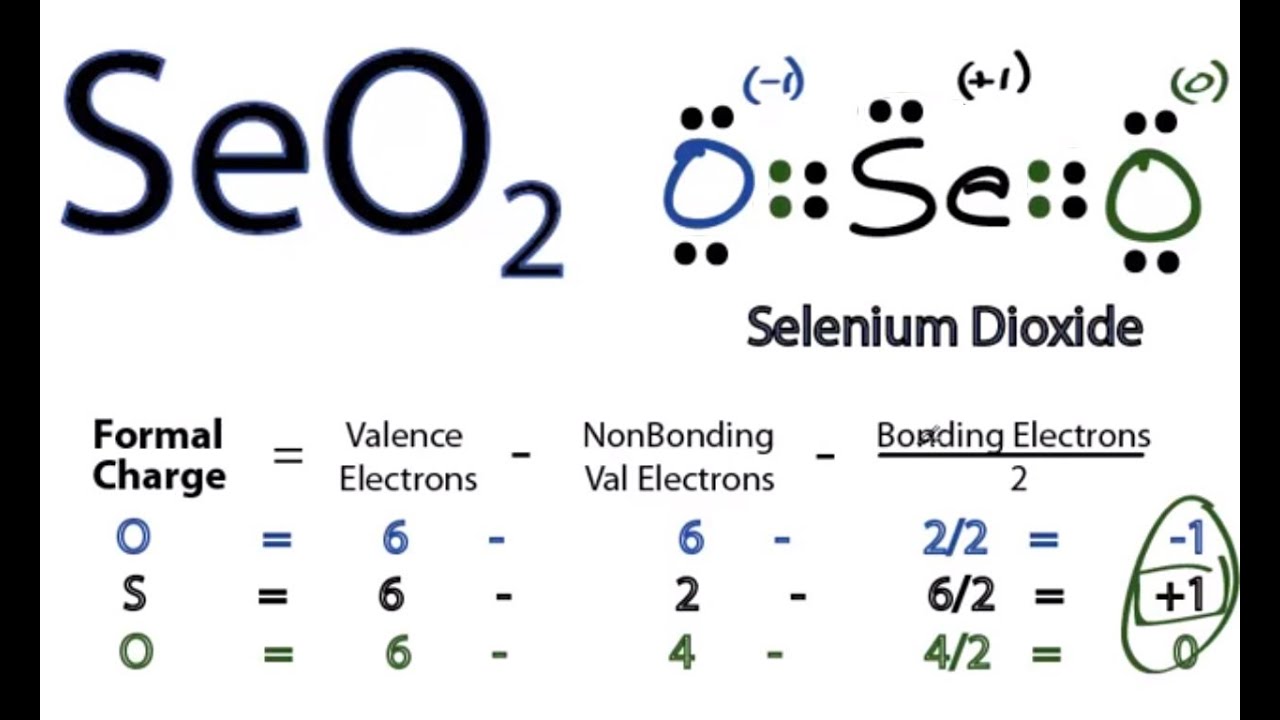
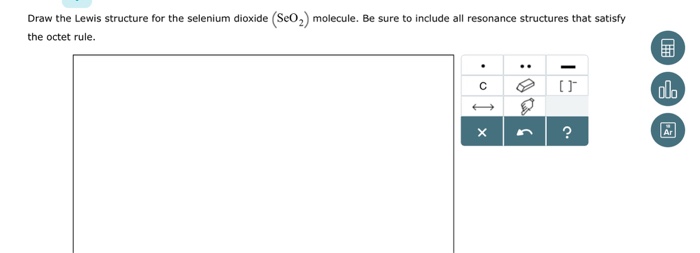
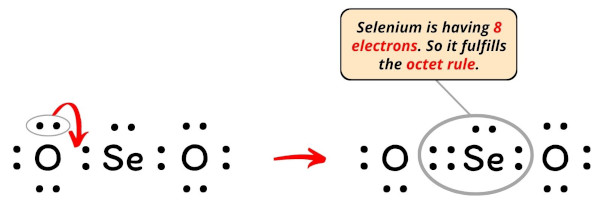
SeO2 Lewis Structure, Geometry, Hybridization, and ... Lewis Structure of Selenium Dioxide (SeO2) Also called the Lewis dot structure, it is a pictorial representation of the behavior and arrangement of valence electrons within an atom. It uses dots to represent valence electrons and lines to show bonds.
6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
How to Draw the Lewis Dot Structure for Se and ... - YouTube A step-by-step explanation of how to draw the Se (Selenium) Se2- Selenide ion) Lewis Dot Structure.For the Se and Se 2- structure use the periodic table to...
Selenium - BASIC INFORMATION Main isotopes of selenium: Se-74, Se-76, Se-77, Se-78, Se-80. Se-79 and Se-82 also exist, but are products of fission. It is a nonmetal. Solid at room temperature. Density- 4.809 g/mL Melting Point- 493.65 K Boiling Point- 958 K Molar Mass- 78.96 g/mol 6 Valence Electrons Electron Configuration- 1s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, 4p4
What is the electron dot structure for selenium? - Answers Sep 14, 2011 · Because selenium is in the 16th column on the periodic chart, it has 6 valence electrons. Thus, the Lewis structure (or electron dot structure) for selenium has 6 dots around it. It will look ...
how many dots would a dot diagram for selenium, one of the ... Because selenium is in the 16th column on the periodic chart, it has 6 valence electrons. Thus, the Lewis structure (or electron dot structure) for selenium has 6 dots around it. It will look similar to this without the underscores: __. . Se: _. . Explanation:
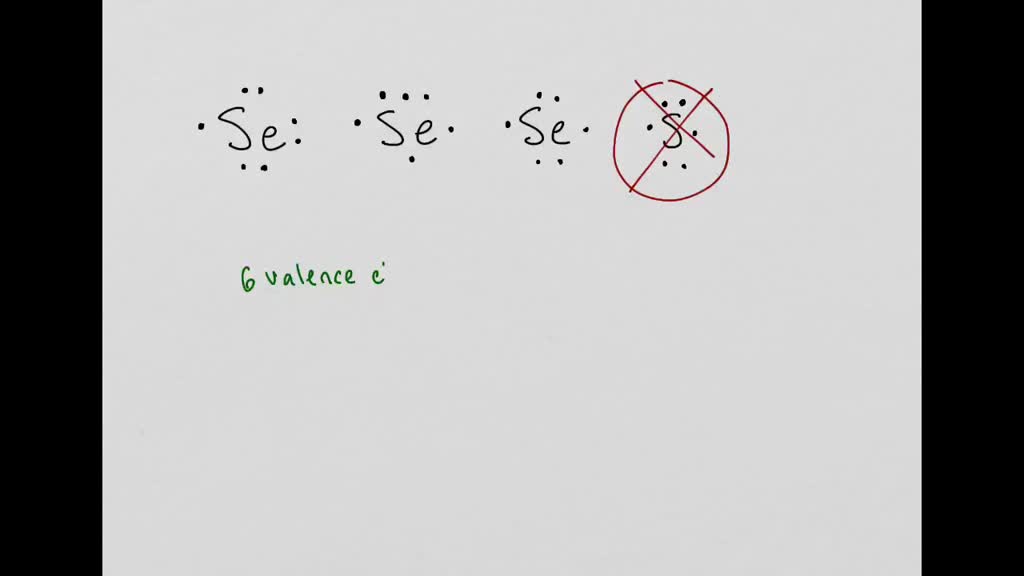
interpret scientific illustrations which is the correct electron dot structure for an atom of seleni
How to Draw the Lewis Dot Structure for SeBr2: Selenium ... A step-by-step explanation of how to draw the SeBr2 Lewis Dot Structure (Selenium dibromide).For the SeBr2 structure use the periodic table to find the total...
Draw the Lewis dot structure for SeO3 and answer the ... Draw the Lewis dot structure for {eq}SeO_3 {/eq} and answer the following questions. ... with each electron domain being between an oxygen atom and the central selenium atom. b. The electron ...
Lewis Diagram For Seo3 Lewis Diagram For Seo3. A Lewis dot structure for SeO3 is drawn with an Se in the center, with two lines connecting it to two Os and one double line connecting it to an O. The Os. Draw all possible resonance structures for the molecule selenium trioxide (SeO3) . The structure and arrows are given, simply add the remaining bonds and lone. (1 ...
Lewis Electron Dot Diagrams - Introductory Chemistry - 1st ... What is the Lewis electron dot diagram for each element? aluminum selenium Solution The valence electron configuration for aluminum is 3 s2 3 p1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons: The valence electron configuration for selenium is 4 s2 4 p4.





















0 Response to "41 selenium electron dot diagram"
Post a Comment