41 xef4 molecular orbital diagram
化學鍵 11 May 2012 — An MO energy level diagram for a AX ... The MOs for XeF4 and the Xe orbitals and LGOs from which they are obtained. AX4 system-1 (D4h). MO.75 pages What is the molecular geometry of XeF3? - FindAnyAnswer.com Therefore, XeF4 molecular geometry is square planar. The bond angles are 90 or 180°. The lone pairs lie on the opposite sides of the molecule basically at 180° from each other. ... All the filled orbitals of Xe have paired electrons. The promotion of one, two or three electrons from the 5p-filled orbitals to the 5d-vacant orbitals will give ...
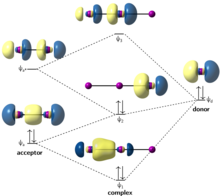
XeF4 Lewis Structure, Molecular Geometry, Hybridization ... MO Diagram of XeF4 An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. When atoms combine with other atoms to make molecules, some of the atomic orbitals adds up to form molecular orbitals which are the same in number.
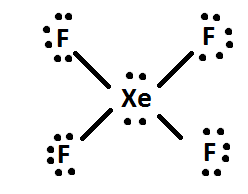
Xef4 molecular orbital diagram
Is XeF4 Polar or Nonpolar? 2021 Beginner's Guide The molecular structure and formation of the Xenon Tetrafluoride can be a basis to verify if XeF4 is a polar or nonpolar molecule. In the chemical compound XeF4, The noble gas central Xe atom reacts with the Fluorine atoms. Four electrons will create bonding orbitals and will be placed on the side of the central atom. XeF4 Molecular Geometry - Science Education and Tutorials The XeF4 molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the XeF4 molecule in a specific geometric manner. Is Xef4 Is A Polar Molecule., Best Overview On: Is Xef4 ... Properties the XeF4 molecule Molecular load of XeF4 molecule is 207.29 g/molDensity the XeF4 molecule is 4.10 g/cm3The Vapour press of the XeF4 molecule is 3mm in ~ room temperature.Bond angle F-Xe-F that XeF4 molecule are 90° or 180° equitorial and also axial place respectively.At room temperature, XeF4 is solid in nature and also boiling allude of XeF4 molecule is 115.7°CMelting point of ...
Xef4 molecular orbital diagram. Solved Q18. (a) Draw the molecular orbital diagram for the ... (a) Draw the molecular orbital diagram for the square planar molecule XeF4. Show the mulliken symbol of orbitals. The ligand group orbitals of this molecule are shown below, from lowest energy to highest energy. LGO's 2&3 are degenerate. Only the radial p orbitals on F are involved in bonding, so don't bother to show the tangential p ... XeF2 Molecular Orbital The lowest vacant orbital, 7σ u, which has an antibonding character consists mainly of 5p z atomic orbitals (AO's) in Xe with a small contribution of 2s and 2p z AO's in two F atoms. The highest occupied valence orbitals, 5π u u, also have an antibonding character and consist of 5p x, y AO's in Xe and 2p x, y in two F atoms. Projection operator method: sigma molecular orbitals of ... Derivation of the sigma molecular orbitals of XeF4 by the projection operator method.0:15 Structure of xenon tetrafluoride1:38 Projection operator table5... DOC VSEPR Theory and Molecular Orbitals VSEPR Theory and Molecular Orbitals VSEPR: Valence Shell Electron Pair Repulsion INSTRUCTIONS: Use your models to predict the molecular geometry for each of the following examples. Fill in the blank space with a sketch of the model you created, and describe the resulting shape in a word or two.
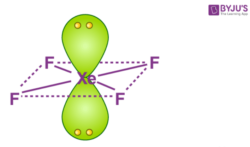
What is the hybridisation and geometry of XeF4 ... What is the molecular shape and polarity for Xenon Tetrafluoride XeF4? As discussed, the XeF4 molecule has a symmetrical square planar shape due to which all the XeF4 bonds have an equal and opposite dipole. Xe and F forms a covalent polar bond due to the difference in electronegativity of both atoms and also result in a net dipole. Hybridization of XeF4: Hybridization of Xe in Xenon ... In the formation of XeF 4, two of the 5p orbital electrons which, in the excited state move to fill the vacant 5 d orbitals. As a result, there are 4 unpaired electrons which include 2 in 5p and 2 in 5d orbitals. This results in sp 3 d 2 hybridization. In the case of fluorine, four F atoms bond with these four half filled orbitals. XeF2 Lewis Structure, Molecular Geometry, Hybridization ... So, the hybridization here is sp3d. Two hybrid orbitals are used for sigma bond formation( single bond) in XeF2 (F-Xe-F). Molecular Orbital Diagram. If we go a little further into chemical bonding and hybridization, we get to know about the Molecular Orbital Theory, a concept of quantum mechanics. Hybridization of XeF4 - Explanation, Structure and ... XeF4 Hybridization. The term 'Hybridization' refers to the formation of newly hybridized orbitals by fusing the atomic orbitals. On the other hand, these newly formed hybridized orbitals affect molecular geometry and bonding properties. Also, the process of hybridization is the development of the valence bond theory.
OneClass: 2. (20 pts) Generate a molecular orbital diagram ... (20 pts) Generate a molecular orbital diagram to accommodate XeF4. For the basis set involving the F-atoms you may worry only about the 2p orbitals pointing towards the central Xe atom. In this way you are only considering Ï -bonding (you can also ignore the F 2S orbitals as these are very stable and don't interact) For the Xe atom basis set ... Solved [10] Q18. (1) Draw the molecular orbital diagram ... (1) Draw the molecular orbital diagram for the square planar molecule XeF4. Show the mulliken symbol of orbitals. The ligand group orbitals of this molecule are shown below, from lowest energy to highest energy. LGO's 2&3 are degenerate. Only the radial p orbitals on F are involved in bonding, so don't bother to show the tangential p orbitals or Chemical bonding Flashcards - Quizlet 59) Draw the molecular orbital diagram shown to determine which of the following is paramagnetic. A) O2^2−. B) Ne2^2+. C) O2^2+. D) F2^2+. E) None of the above are paramagnetic. D) F2^2+. 60) Draw the molecular orbital diagram shown to determine which of the following is most stable. A) C2^2+. Number of hybrid orbitals in XeF4 is: >> Number of hybrid orbitals in XeF4 is: ... Hybridization is highly correlated with molecular shapes or inter orbital angles. If there is any departure in geometry (on the basis of VSEPR theory), the apparent departure in hybridization can be observed and the following characteristic relationship is the easiest way to interpret that departure.
Xef4(Xenon Tetrafluoride) Molecular Geometry, Lewis ... But when this atom is in an excited state, two electrons in the p-orbitals move to d-orbitals; as a result, there are four unpaired electrons in total. Out of which, two are in p-orbitals, and the other two unpaired electrons are in d-orbitals. These hybridized orbitals lead to sp3d2 hybridization in XeF4. XeF4 Molecular Geometry
Final Exam - nmsu.edu is a Web Hosting 9 Dec 2011 — c) (15 points) Construct a molecular orbital diagram for XeF4 that includes four valence atomic orbitals (AOs) for all five atoms, assuming that ...6 pages
Projection operator method: sigma molecular orbitals of ... Derivation of the sigma molecular orbitals of XeF4 by the projection operator method.00:15 Structure of xenon tetrafluoride03:08 Reducible representation...
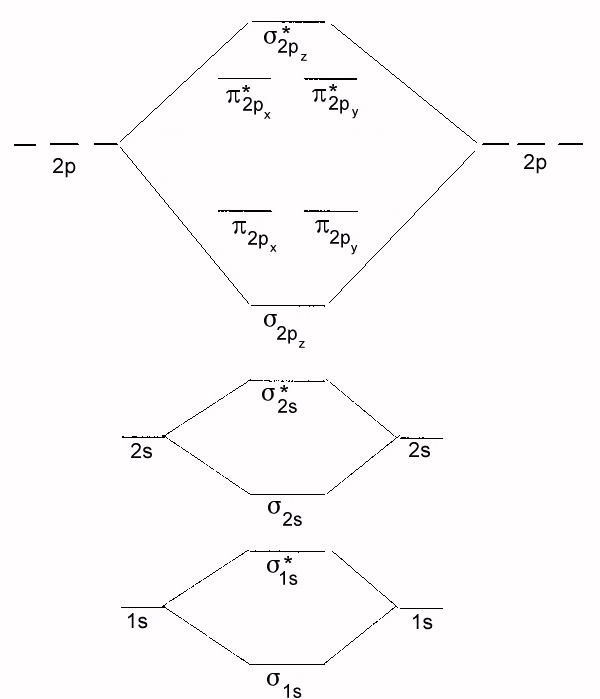
PDF MO Diagrams for Diatomic Molecules Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Molecular Orbital Diagram Maker ©2022 Prof Adam J Bridgeman | close window : ©2022 Prof Adam J Bridgeman | close windowProf Adam J Bridgeman | close window
patapum.to.itFlour Mill Rye [4MH368] Search: Rye Flour Mill. What is Rye Flour Mill. Every flour has its own unique properties. Sourdough Rye using your flour and some crushed organic caraway seeds has lifted my Sourdough Rye to a new level!!
xef4 molecular orbital diagram - pem.pm An MO diagram is a descriptive instrument that is particularly used to explain the formation of chemical bonds in molecules with the help of molecular orbital theory. Dear Student, a) b)In,XeF4,the central atom,Xe,has eight electrons in its outermost shell. Determine whether each is paramagnetic or diamagnetic.
Semi-empirical molecular orbital energy levels of XeF4 ... Using recently published analytical SCF wave functions for xenon, the one-electron molecular orbital energies of xenon tetrafluoride have been re-determined in the Wolfsberg-Helmholz semi-empirical approximation. Unlike similar previous investigations, the present study takes into consideration ligand-ligand overlap, and uses the recently proposed reciprocal mean for the semi-empirical ...
Q18. (a) Draw the molecular orbital diagram for the square ... Q18. (a) Draw the molecular orbital diagram for the square planar molecule XeF4. Show the mulliken symbol of orbitals. The ligand group orbitals of this ...4 answers · Top answer: girls we want Thio draw the lewis dot structure, determined the geometry to determine whether ...
Is Xef4 Polar or NonPolar? - textilesgreen Xef4 polar or nonpolar molecules - xef4 is nonpolar molecules because the electronegativity is different between xe and f4. f4 has more electronegativity then xe. Hello, reders welcome to " textilesgreen.in " today we will discuss about xef4 polar or nonpolar, molecular geometry for xef4, xef4 polar and more.
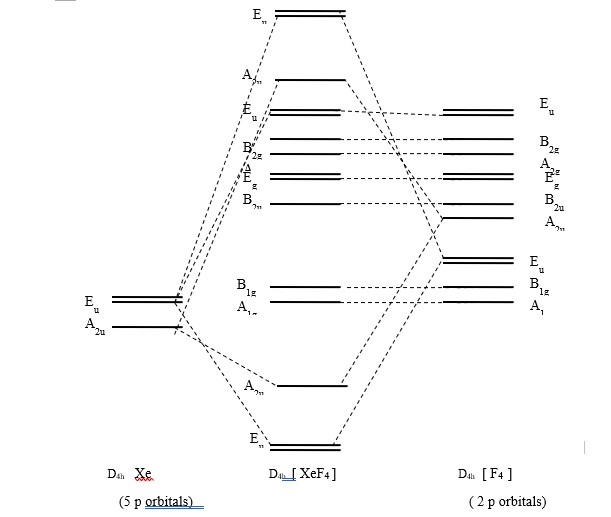
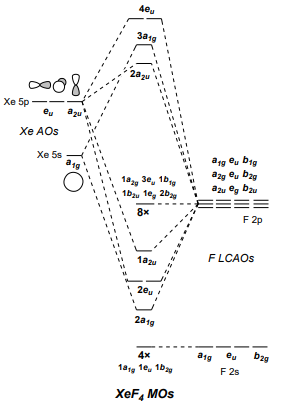
PDF Inorganic Chemistry with Doc M. - Creighton University Molecular orbital diagram for square planer XeF4 6. Using p-orbitals for σ-bonding: molecular orbital diagram for trigonal planer BF3 1. Symmetry adapted linear combinations (SALC) of bonding group atomic orbitals For molecules where the central atom is bonded to more than one other B group such as the
CH 10 Bonding & Molecular Structure: Orbital Hybridization ... CH 10 Bonding & Molecular Structure: Orbital Hybridization & Molecular Orbitals. a) MO theory predicts that electrons are delocalized over the molecule. b) VB theory predicts that oxygen is paramagnetic, MO theory does not. c) VB theory describes a molecular bond as the overlap between two atomic orbitals.
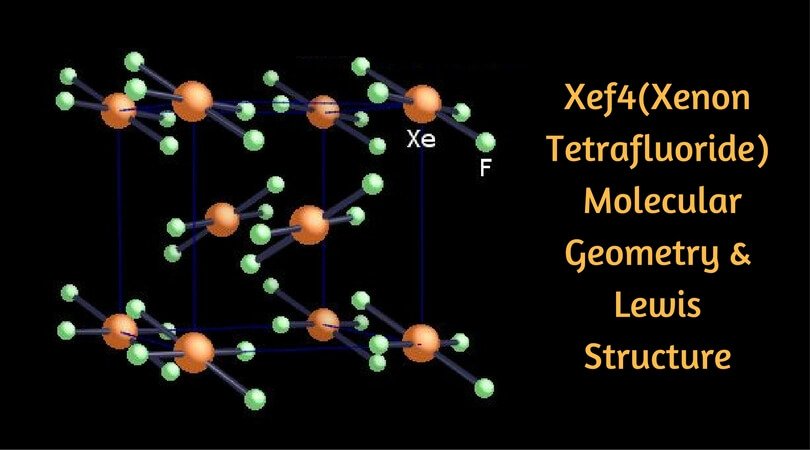
Xenon tetrafluoride (XeF4) - D4h Symmetry — ChemTube3D Click the Symmetry Operations above to view them in 3D. XeF 4 belongs to the D 4h Point group and contains; One C 4 rotation axis, one C 2 rotation axis (equivalent to C 42 ), Four C 2 axes perpendicular to the C 4 axis. 4σ planes of symmetry,one σ h plane. One S 4 axis.
What is the shape molecular geometry of XeF4? - Book Vea Xenon tetrafluoride XeF4 compound has a planar structure. In XeF4 the 'Xe' atom is sp3 d2 hybridised, which contains two lone pair orbitals and four bond pair orbitals. Therefore the shape of the XeF4 molecule is square planar. with one lone pair orbital over and the other below the plane.
xef4 sigma and pi bonds - girlcrush.us The number of pi bonds in the molecule below is. Answer. Sigma bonds are formed by end-to-end overlapping and Pi bonds are when the lobe of one atomic orbital overlaps another. Share SlideShare. Xef4(xenon tetrafluoride) molecular geometry lewis xenon tetrafluoride of xef2 無料ダウンロード f structure サゾナタメ draw for xef4.
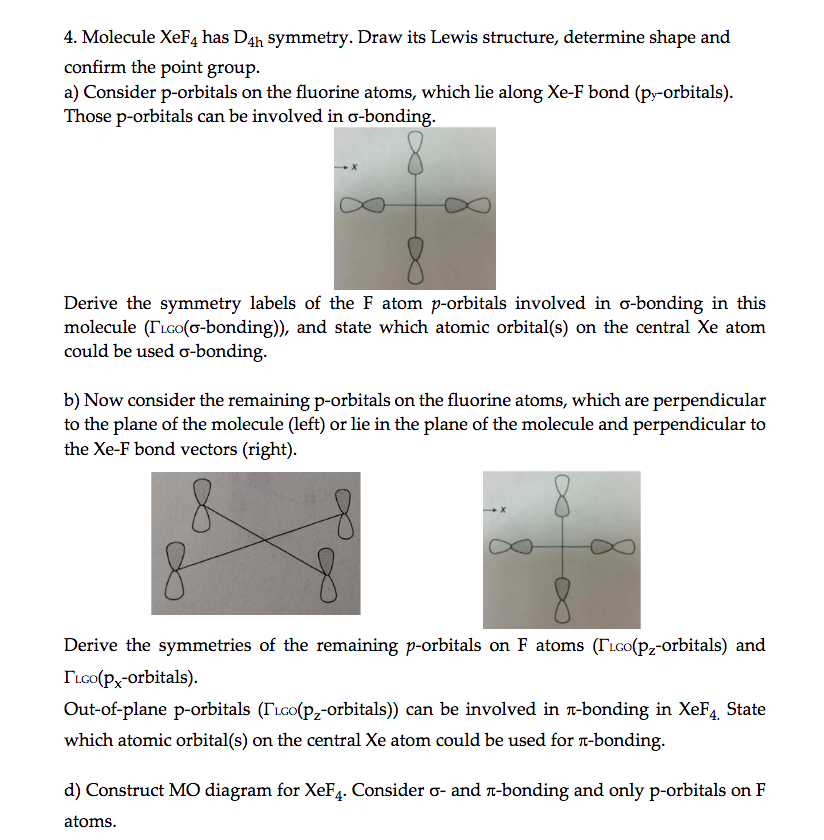
Molecule XeF4 has D4h symmetry. d) Construct MO ... Use symmetry analysis to generate a molecular orbital diagram for XeF4. As a basis set, consider one σ-bonding hybrid on each F that can be treated thesame ...
quizlet.com › 477461106 › chemmy-flash-cardschemmy Flashcards - Quizlet The predicted molecular shape of PH3 according to the VSEPR theory is ... The correct orbital diagram for the valence electrons of silicon is ... If 103.5 g of XeF4 ...
Is Xef4 Is A Polar Molecule., Best Overview On: Is Xef4 ... Properties the XeF4 molecule Molecular load of XeF4 molecule is 207.29 g/molDensity the XeF4 molecule is 4.10 g/cm3The Vapour press of the XeF4 molecule is 3mm in ~ room temperature.Bond angle F-Xe-F that XeF4 molecule are 90° or 180° equitorial and also axial place respectively.At room temperature, XeF4 is solid in nature and also boiling allude of XeF4 molecule is 115.7°CMelting point of ...
XeF4 Molecular Geometry - Science Education and Tutorials The XeF4 molecular geometry is a diagram that illustrates the number of valence electrons and bond electron pairs in the XeF4 molecule in a specific geometric manner.
Is XeF4 Polar or Nonpolar? 2021 Beginner's Guide The molecular structure and formation of the Xenon Tetrafluoride can be a basis to verify if XeF4 is a polar or nonpolar molecule. In the chemical compound XeF4, The noble gas central Xe atom reacts with the Fluorine atoms. Four electrons will create bonding orbitals and will be placed on the side of the central atom.
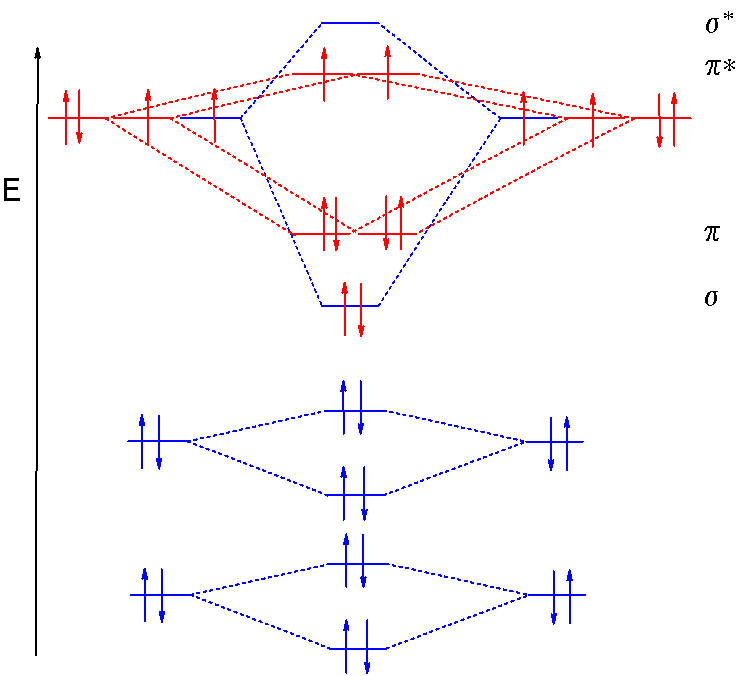

![MO diagram of T d [CsO 4 ] + , as interaction between a Cs + ...](https://www.researchgate.net/publication/317849955/figure/fig2/AS:614161100836879@1523438825309/MO-diagram-of-T-d-CsO-4-as-interaction-between-a-Cs-ion-and-an-O-4-fragment.png)

![Solved 4. [26 Pts] Complete the following exercises related ...](https://media.cheggcdn.com/media/75b/75b284b8-383e-478d-9eea-a89d4f9d1bc6/phpJz56EY.png)




![Solved [10] Q18. (1) Draw the molecular orbital diagram for ...](https://media.cheggcdn.com/media/4b1/4b19a3d6-46d0-4882-a3dd-9df20a52de3f/phpJjbLS0)






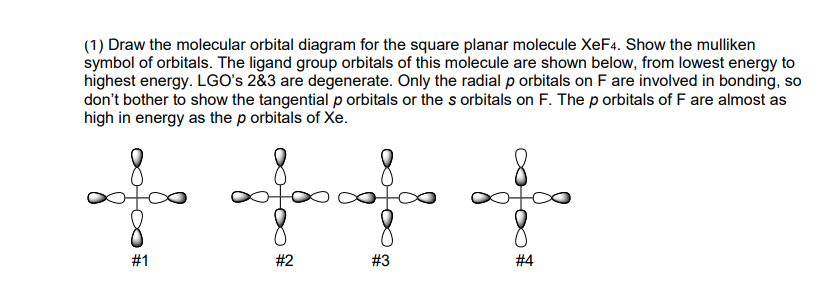
![SOLVED:[10] Q18. Draw the molecular orbital diagram for the ...](https://cdn.numerade.com/ask_images/c643cbd225bb4763b0d50dd9e5cd477c.jpg)



0 Response to "41 xef4 molecular orbital diagram"
Post a Comment