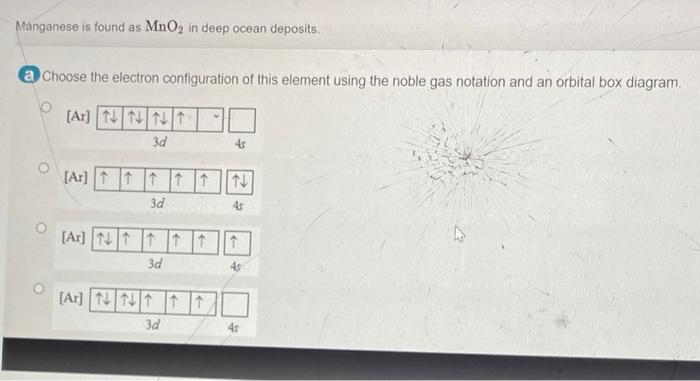
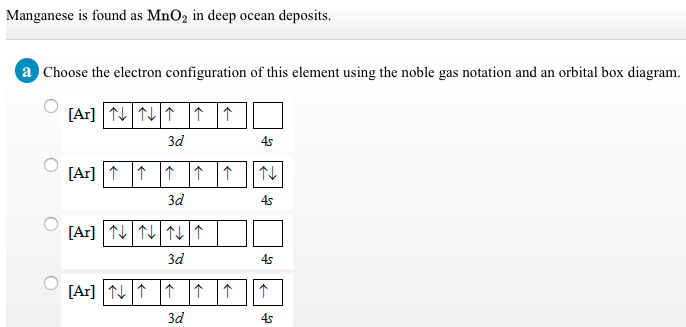
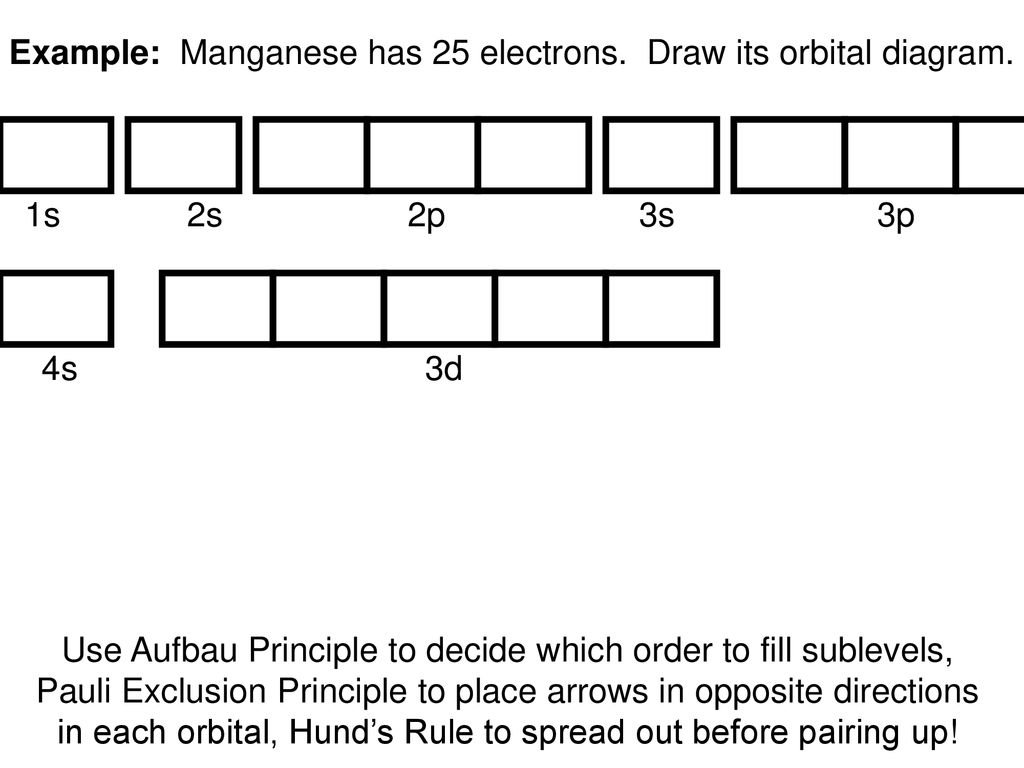
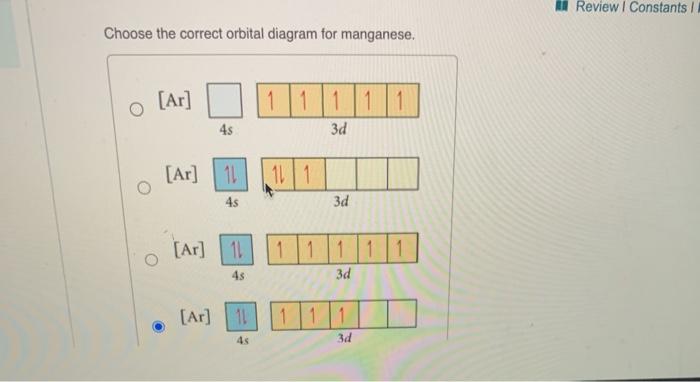
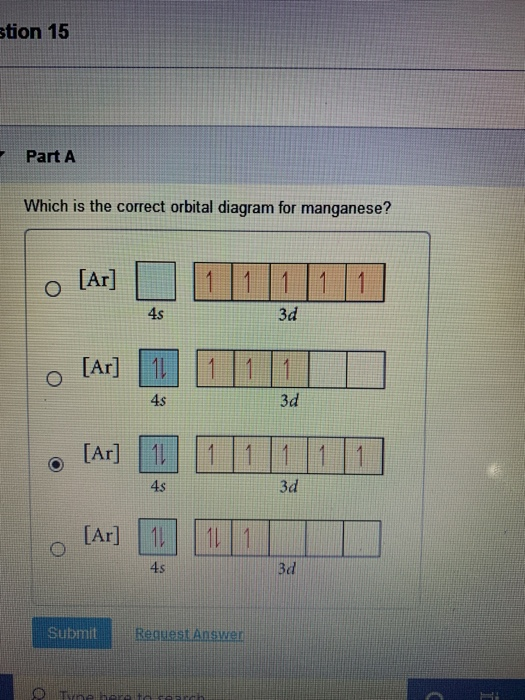
44 choose the correct orbital diagram for manganese.
Chemistry Archive | June 01, 2021 | Chegg.com Choose the best answer The bond length in H'C135 is 1.0*10-10 m. The position of fundamental peak is 2899.3 cm ?. Calculate the position of line 4 in R-branch (cm). 40 choose the correct orbital diagram for vanadium ... 37) The orbital diagram for fluorine shows 1 unpaired electron in a p orbital. 38) The correct electron configuration for magnesium is: 1s 2 2s 2 2p 6 3s 3. 39) The element manganese (symbol = Mn) has five valence electrons.
Chapter 9 electrons in atoms and the periodic table 100% ... 37) The orbital diagram for fluorine shows 1 unpaired electron in a p orbital. 38) The correct electron configuration for magnesium is: 1s 2 2s 2 2p 6 3s 3. 39) The element manganese (symbol = Mn) has five valence electrons. 40) Bromine has 17 valence electrons. 41) Bromine has 28 core electrons.

Choose the correct orbital diagram for manganese.
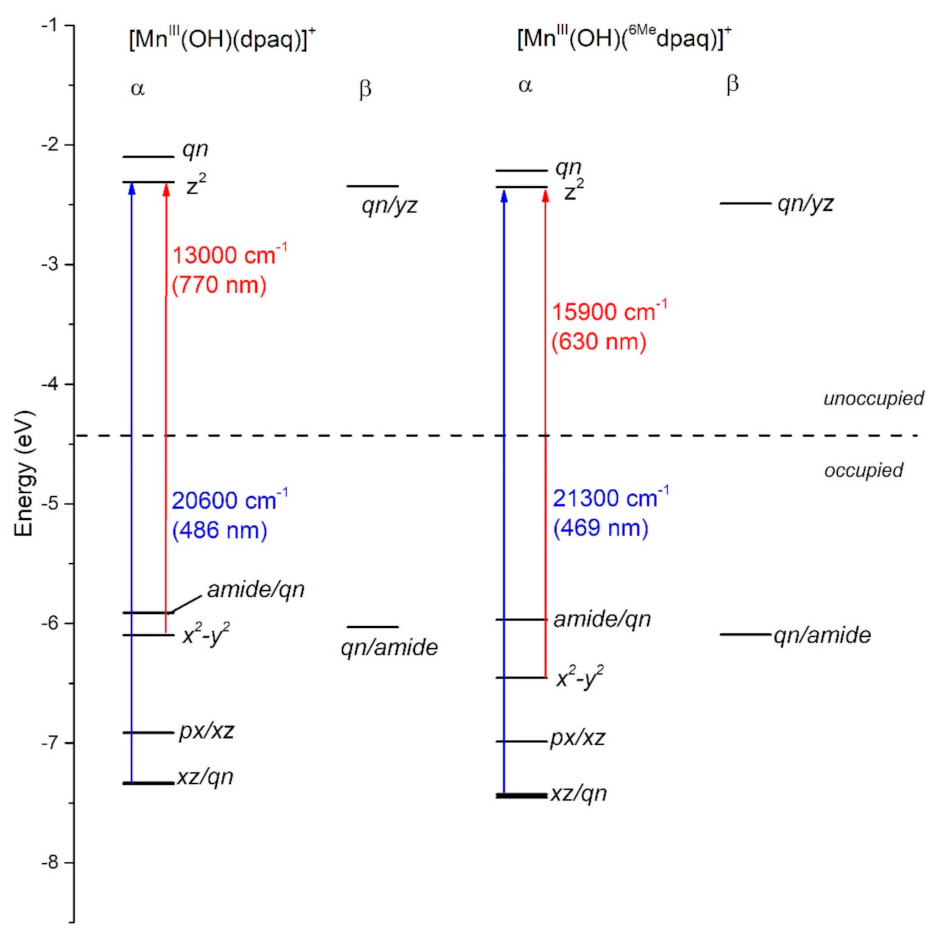
Titanium oxide and chemical inhomogeneity in the ... For this toy planet it is assumed that the gradient in the tested species causes an apparent orbital velocity of 150 km s −1. Right: a general day-to-nightside gradient and global flow ... NCERT Exemplar Class 11 Chemistry Unit 2 Structure of Atom Choose the correct option out of the choices given below each question. Assertion (A) : All isotopes of a given element show the same type of chemical behaviour. Reason (R) : The chemical properties of an atom are controlled by the number of electrons in the atom. (i) Both A and R are true and R is the correct explanation of A. Crystal Field Theory - Amrita Vishwa Vidyapeetham Orbital Splitting: The five d-orbitals are given the symbols dxy, dzx, dyz, dx 2-y 2 and dz 2. In a complex they are all differently aligned relative to the incoming charge. Depending on the geometry of the complex, some of the d-orbitals will point directly towards the ligands, while some will point between them.
Choose the correct orbital diagram for manganese.. Chemistry Semester-2 ICSE Specimen Paper Solved Class-10 ... (a) Manganese dioxide reacts with concentrated HCl. (b) A glass rod dipped in concentrated HCl acid is brought near ammonia gas. (c) Concentrated sulphuric acid is added to carbon. Answer : (iv) Write balanced equation for the following conversions: (a) Lead sulphate from lead nitrate and sulphuric acid. (b) Nitrogen tri chloride from ammonia. What Is The Electron Configuration For The Fe3+ Ion? Ok. There is only one unpaired electron in 3d orbital which contains only 5 electrons. 1s2, 2s2, 2p6, 3s2, 3p6, 3d5. Two electrons are removed from 4s orbital and one electron is removed from 3d orbital. Chemistry Archive | May 24, 2021 | Chegg.com The uranyl ion, [UO2)2+, is a ubiquitous structural motif in U (VI) chemistry. The molecule is linear and the U-O bond length is approximately 28. When lying along the z-axis, the coordinates of ur. b Write a mechanism for the reaction using curved arrows to show electron reorganization. Atomic Number & Mass Number - Definition, Facts, Videos ... Atomic Number Orbital Energy Levels. When an electron is at a specific energy level, it is more likely to be found in certain portions of that level than others. Orbitals are the name for these sections. Sublevels are made up of orbitals with the same energy. A maximum of two electrons can be found in each orbital.
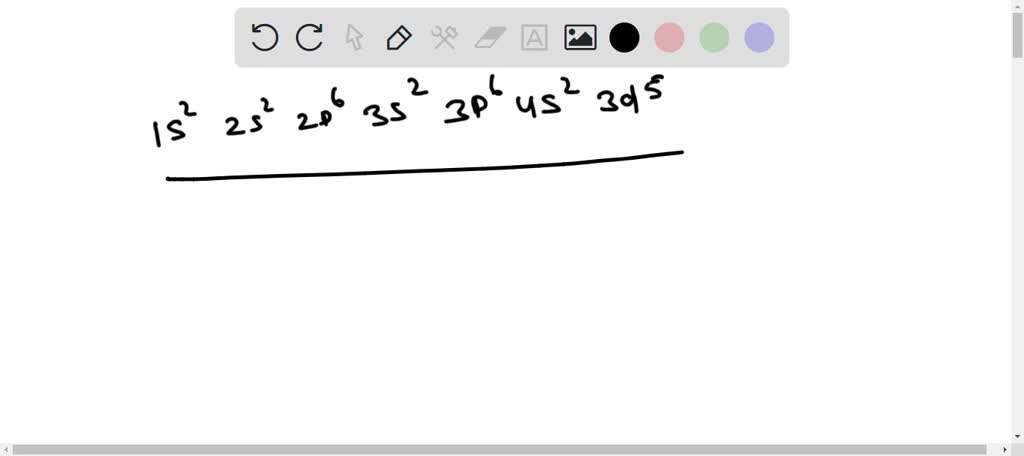
Electron configurations of the elements (data page ... Main article: Electron configuration. This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise form, then with all subshells written out, followed by the number of electrons per shell. Electron configurations of elements beyond hassium (element 108 ... Chem 103 Module 1 to 6 Exam answers Portage learning ... Chem 103 Module 1 to 6 Exam answers Portage learning. MODULE 1 EXAM Question 1 Click this link to access the Periodic Table. This may be helpful throughout the exam. 1. Convert 845.3 to exponential form and explain your answer. 2. Convert 3.21 x 10-5 to ordinary form and explain your answer. Question 2 C The Electron Configuration: Ions Video & Text Solutions ... Our videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams. Valence Electrons Chart for All Elements (Full Chart Inside) Valence electrons in Manganese (Mn) 7: 26: Valence electrons in Iron (Fe) 8: 27: Valence electrons in Cobalt (Co) 9: 28: Valence electrons in Nickel (Ni) 10: 29: Valence electrons in Copper (Cu) 11: 30: Valence electrons in Zinc (Zn) 12: 31: Valence electrons in Gallium (Ga) 3: 32: Valence electrons in Germanium (Ge) 4: 33: Valence electrons in ...
Angular Momentum Quantum Number: Definition & Example ... Angular Momentum Quantum Number. There are four quantum numbers that make up the address for an electron. Of the four quantum numbers, our focus for this lesson is the angular momentum quantum ... Chapter 9 electrons in atoms and the periodic table 100% ... Chapter 9 Electrons in Atoms and the Periodic Table 9.1 True/False Questions 1) When the elements are arranged in order of increasing number of protons, certain sets of properties recur periodically. 2) The early scientists who developed the quantum-mechanical model were bewildered by the model and it altered our fundamental view of matter. 3) Light is a type of […] Maharashtra Board Class 12 Chemistry Solutions Chapter 9 ... Table 9.5 : d-orbitai diagrams fir high spin and low spin complexes (Only the electronic configurations c4 to d1 render the high spin and low spin complexes) Question viii. [CoCl 4] 2⊕ is a tetrahedral complex. Draw its box orbital diagram. State which orbitals participate in hybridization. Answer: 27 Co [Ar] 3d 7 4s 2 Spin Quantum Number: Definition & Example - Study.com Spin Quantum Number. Let's think about an electron in an atom; first, imagine a spinning top. A top can spin clockwise or counter-clockwise. In the same way, an electron occupying an orbital ...
Electron Arrangement In Atoms Worksheet Answers What is not as intuitive is why the size decreases from left to right. Orbital representation diagram for potassium, or choose one as random, regions of space if an atom where two specific electrons are add likely to other found. However this worksheet above. Because half their differing nuclear charges, electrons are grouped into orbitals, or pz.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital Diagram of All Elements (Diagrams given Below) January 1, 2022 April 10, 2021 by Admin Orbital diagrams (Orbital box diagrams) of all elements are mentioned in the chart given below.
What Is The Complete Ground State Electron Configuration ... Then, continue writing the electron configuration of a certain element until you reach the correct number of electrons. Answer and Explanation: The electron configuration of titanium is 1s2 2s2 2p6 3s2 3p6 4s2 3d2. Titanium is the second element in the d-block, which consists of transition metals. ... Each orbital has a specific energy ...
Chapter 9 - Proteins and Enzymes - CHE 120 - Introduction ... This helix is stabilized by intrachain hydrogen bonding between the carbonyl oxygen atom of one amino acid and the amide hydrogen atom four amino acids up the chain (located on the next turn of the helix) and is known as a right-handed α-helix. X ray data indicate that this helix makes one turn for every 3.6 amino acids, and the side chains of ...
Chapter 4 - Atoms, Molecules, and Ions - CHE 105/110 ... If each green ball represents a sulfur atom, then the diagram on the left represents an S 8 molecule. The molecule on the right shows that one form of elemental phosphorus exists, as a four-atom molecule.
Chem 103 Module 1 to 6 Exam answers Portage learning ... Chem 103 Module 1 to 6 Exam answers Portage learning. MODULE 1 EXAM Question 1 Click this link to access the Periodic Table. This may be helpful throughout the exam. 1. Convert 845.3 to exponential form and explain your answer. 2. Convert 3.21 x 10-5 to ordinary form and explain your answer. Question 2 Click this link to access the Periodic Table. This may be helpful throughout the exam. Using ...
how many valence electrons does vanadium have The Aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals, but this is actually not true for most elements! The valence electrons of these elements fall under the d orbital. Manganese has a complex structure which has not been included in this study, whereas Fe (3 d 6 4 s 2 ) has a b.c.c.
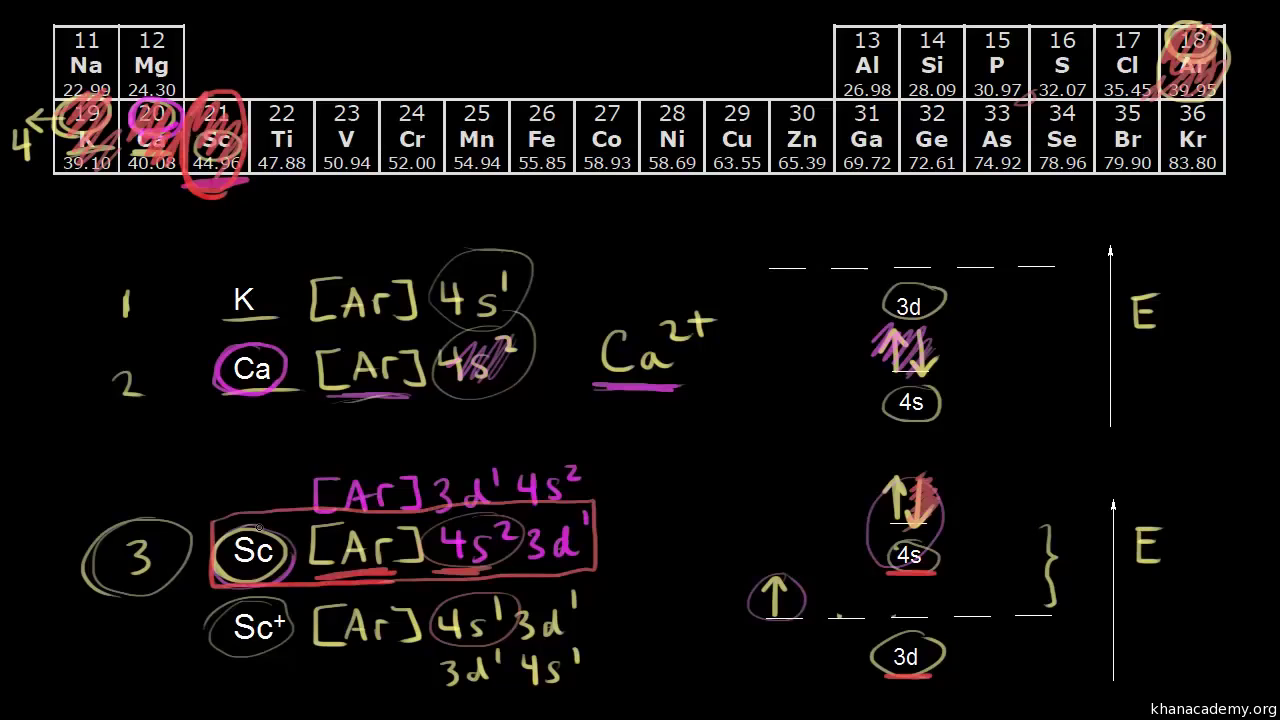
Manganese 2 Ion Electron Configuration / How Many 3d ... Put the diagrams of electronic configurations into the correct order to show the progression of an mn atom to an mn²⁺ ion. Manganese has seven ionic forms from mn(i) to mn(vii). For mn2+ i would say 1s2 2s2 2p6 3s2 3p6 4s0 3d5 because the electrons are always removed from the 4s orbital first.
Important Questions - Page 19 - Maharashtra Board Solutions (d) Both A and R are correct but R is not the correct explanation of A. Answer: (d) Both A and R are correct but R is not the correct explanation of A. Question 5. A - Higher sea levels may lead to deadlier cyclones and also frequent flooding of coastal areas. R - High precipitation leads to higher sea level. (a) Only A is correct.
what is the value of the principal quantum number ... What is the correct values for N and L for the orbital 5d? n=5 and ml=2. Which of the following set of quantum numbers is correct for an electron in 5d orbital? So the only possible set of quantum numbers for an electron in 5d orbital from the given options is: n=5,l=2,m=2,s=+12. What are all the possible values of ML If L 1 AP orbital )?
What Is The Electron Configuration For Rb? [Comprehensive ... The electronic configuration of the element with atomic number 35 is 1s22s22p63s23p63d104s24p5 the last valence electrons enter into p orbital. Which element is 1s2 2s2 2p6 3s2 3p6 4s2 3d3? Vanadium Example: 1s2 2s2 2p6 3s2 3p6 4s2 3d3 (This has a total of 23 electrons which equals the atomic number of V - Vanadium.)
Crystal Field Theory - Amrita Vishwa Vidyapeetham Orbital Splitting: The five d-orbitals are given the symbols dxy, dzx, dyz, dx 2-y 2 and dz 2. In a complex they are all differently aligned relative to the incoming charge. Depending on the geometry of the complex, some of the d-orbitals will point directly towards the ligands, while some will point between them.
NCERT Exemplar Class 11 Chemistry Unit 2 Structure of Atom Choose the correct option out of the choices given below each question. Assertion (A) : All isotopes of a given element show the same type of chemical behaviour. Reason (R) : The chemical properties of an atom are controlled by the number of electrons in the atom. (i) Both A and R are true and R is the correct explanation of A.
Titanium oxide and chemical inhomogeneity in the ... For this toy planet it is assumed that the gradient in the tested species causes an apparent orbital velocity of 150 km s −1. Right: a general day-to-nightside gradient and global flow ...















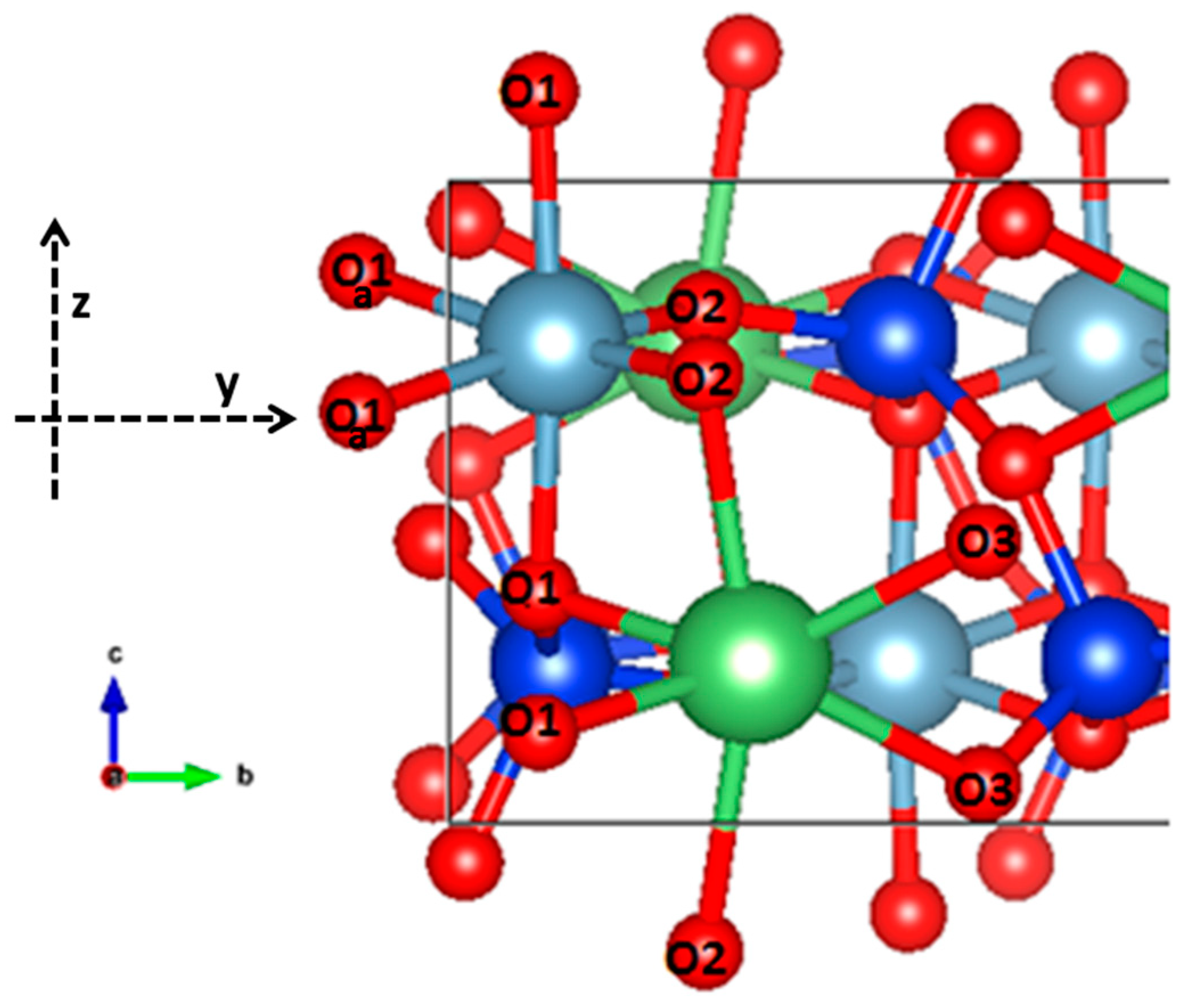
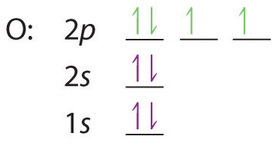






0 Response to "44 choose the correct orbital diagram for manganese."
Post a Comment