44 d orbital splitting diagram
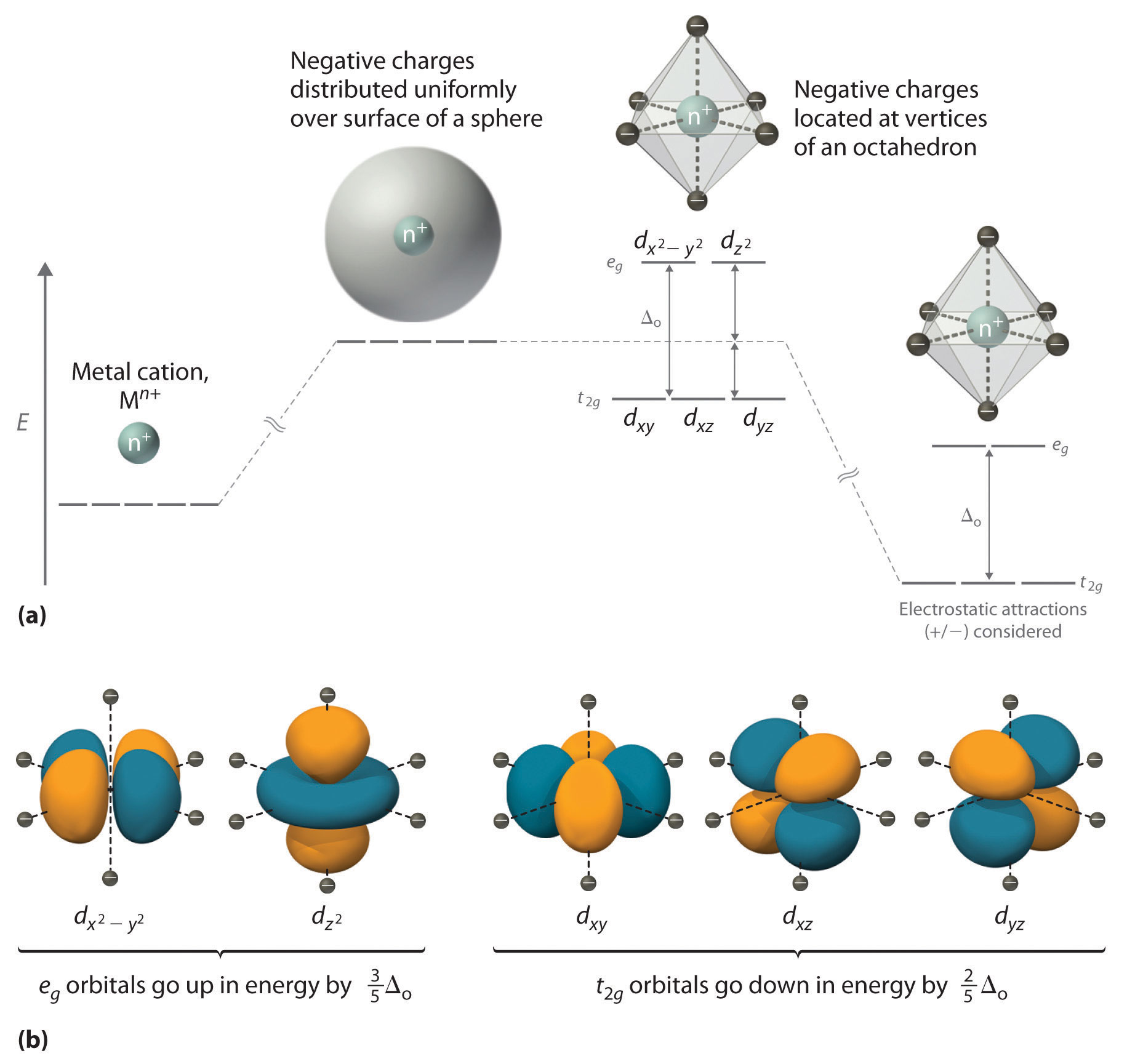
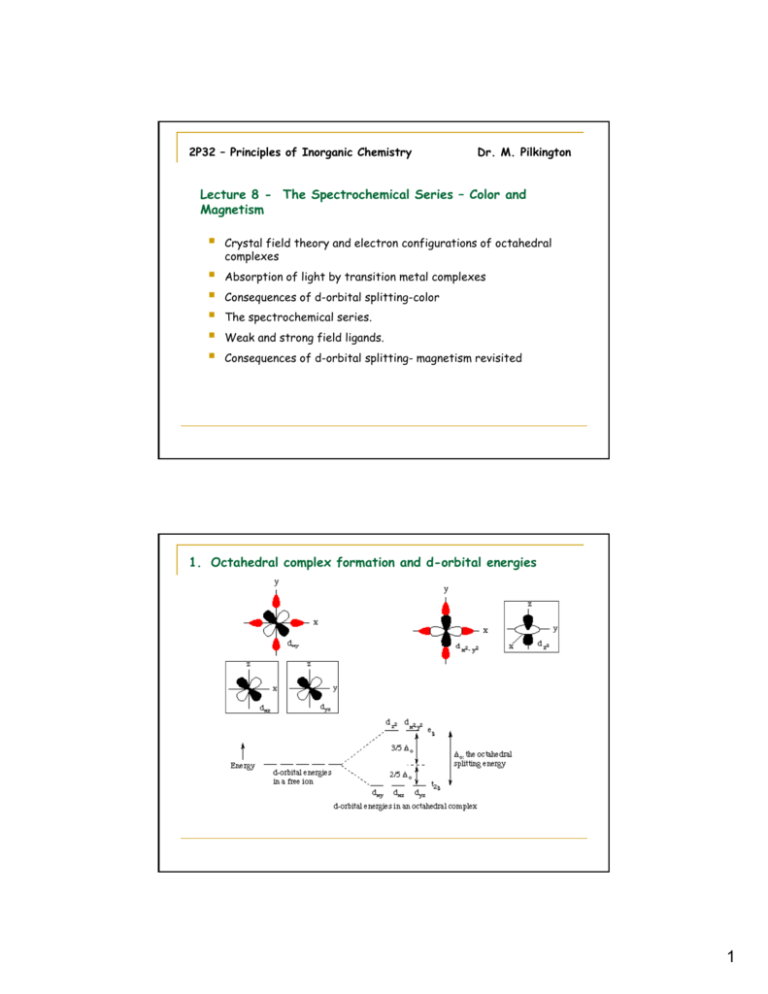
D. Consequences of d-Orbital Splitting: Colour This is called d-orbital splitting. Consider the 5 d-orbitals in an xyz coordinate system. The combination of two orbitals produces the unique dz2 orbital: B. Splitting of the d-Orbitals in an Octahedral d-orbital diagram for [Fe(CN)6]3-. The first three electrons go into t2g orbitals as before. How do p-orbitals and d-orbitals split in an octahedral crystal field? You can calculate crystal field splitting energy by using above diagram for various complexes with six number of ligands present. When ligands approach any orbital, there is repulsion but not equally in all orbitals. Some orbitals which are located at a direct angle from the field of ligand face more...
ions - How to determine the greatest d orbital splitting? - Chemistry... Which complex has the greatest d orbital splitting? $\begingroup$ @AnthonyP The splitting of the d orbitals is not only a function of the ligand, but also the metal (the atom type as well as the oxidation state).
D orbital splitting diagram
Crystal Field Theory | d-Orbital Splittings d-Orbital Splittings. CFT focuses on the interaction of the five (n − 1)d orbitals with ligands arranged in a regular array around a transition-metal ion. We can use the d-orbital energy-level diagram in Figure The splitting of the d orbitals because of their interaction with the ligands in a complex has... d-Metal Complexes This theory explains the splitting of the d orbitals to remove their degeneracy, the number of unpaired electrons in transition metal complexes, their color, spectra and magnetic properties. In this case, the dz2 orbital drops even lower in energy, and the molecule has the following orbital splitting diagram. Answered: PRE-LAB QUESTION After consulting… | bartleby Mar 17, 2022 · PRE-LAB QUESTION After consulting sections 5.7 of your text, draw the complete MO diagram for the diatomic molecule, H2. Next to each of the MOs in your diagram, draw a picture that describes the constructive or destructive interference of atomic wave functions and indicate the location of any nodes.
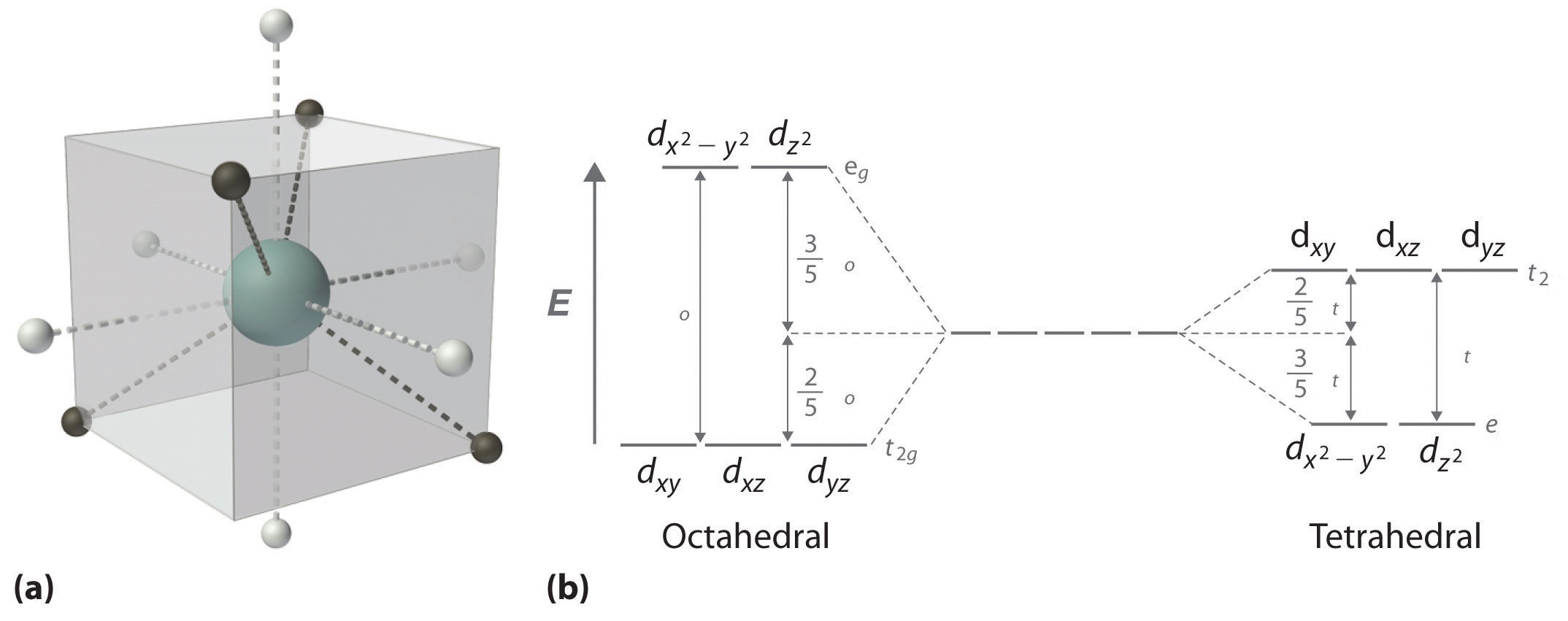
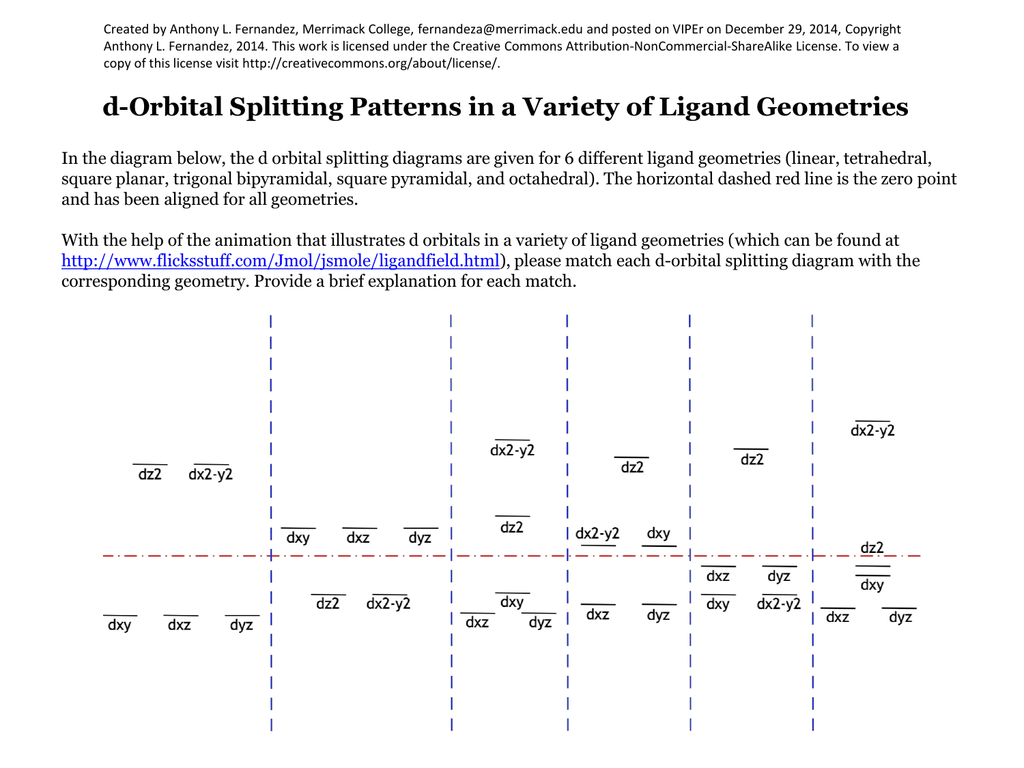
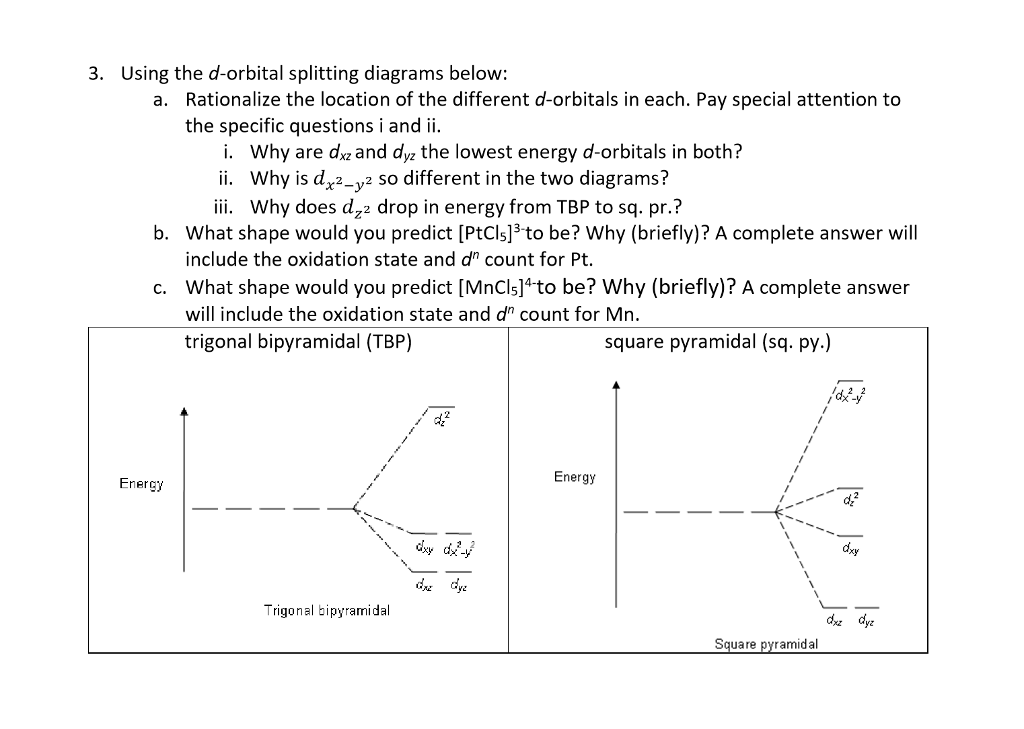
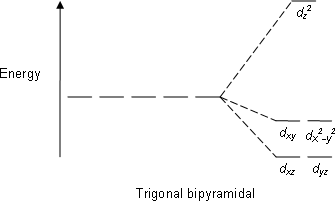
D orbital splitting diagram. D-orbital splitting diagrams | Physics Forums Homework Statement Sketch d-orbital splitting diagrams for a complex in Td symmetry and a complex in D4h symmetry. Label the orbitals as bonding... Introduction to Inorganic Chemistry/Coordination Chemistry and Crystal... Derive the d-orbital splitting patterns for octahedral, elongated octahedral, square pyramidal, square planar, and tetrahedral complexes. For octahedral and tetrahedral complexes, determine the number of unpaired electrons and calculate the crystal field stabilization energy. Fig. 6 (a) Qualitative d-orbital splitting diagram and electron... Download scientific diagram | (a) Qualitative d-orbital splitting diagram and electron configuration for [Fe(C 3 S 5 ) 2 ] 2À with q d ¼ 90 . (b) Tilted coordinate axes and graphical depiction of the d xz and d yz orbitals of the iron(II) ion with reference to the S 3p orbitals of the C 3 S 5 2À lowest unoccupied... d-Orbital Splitting Patterns in a Variety of Ligand Geometries | VIPEr In this activity, the provided d orbital splitting patterns need to be matched with ligand geometries. Students are provided with the d orbital splitting diagrams for 6 ligand geometries (octahedral, trigonal bipyramidal, square pyramidal, tetrahedral, square planar, and linear).
Sodium Atomic Emission Spectrum - University of California ... of the influences which cause splitting of the emission lines of atomic spectra. The transition which gives rise to the doublet is from the 3p to the 3s level, levels which would be the same in the hydrogen atom. The fact that the 3s state (total orbital angular momentum quantum number L = 0) is lower than the 3p state (L=1) is Chapter 2 structure of atom class 11 - SlideShare Jun 30, 2015 · d- orbitals The 5 d-orbitals are designated as:- The shapes of the first four d-orbitals are similar to each other , where as the fifth one is different form others, but all 5 have 3d- orbitals and are equivalent in energy. The d- orbitals for which n is greater than 3 also have shapes similar to 3d orbital , but differ in energy. Covalent Bonds vs Ionic Bonds - Difference and Comparison ... For example, let us consider a Methane molecule i.e.CH 4. Carbon has 6 electrons and its electronic configuration is 1s22s22p2, i.e. it has 4 electrons in its outer orbit. According to the Octate rule ( It states that atoms tend to gain, lose, or share electrons so that each atom has full outermost energy level which is typically 8 electrons.), to be in a stable state, it needs 4 more … The Zeeman Effect - Physics Courses splitting in a magnetic field for the 2P 3>2, 2P 1>2, and 2S 1>2 energy levels for sodium, showing the anomalous Zeeman effect. These are the D 1 and D 2 lines in Figure 7-22. The splitting of the levels depends on L, S, and J, leading to more than the three lines seen in the normal effect. [Photo from H.E. White, Introduction to Atomic
In the diagram below, the d orbital splitting diagrams are given for 6 With the help of the animation that illustrates d orbitals in a variety of ligand geometries (which can be found at ), please match each d-orbital splitting diagram with the corresponding geometry. Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine … Lecture 10 -Further Consequences of d-Orbital Splitting The resulting d-orbital splitting diagram for tetrahedral coordination is the inverse of the diagram for octahedral coordination, as shown below. 8 (ii) Square Planar Complexes d-Orbital Splitting in Square Planar Coordination. Square planar coordination can be imagined to result when two ligands... Crystal Field Theory - Chemistry LibreTexts Crystal field theory (CFT) describes the breaking of orbital degeneracy in transition metal complexes due to the presence of ligands. There is a large energy separation between the dz² orbital and the dxz and dyz orbitals, meaning that the crystal field splitting energy is large.
Orbital splitting diagram - Big Chemical Encyclopedia Orbital splitting diagrams for dP complexes with elongated and compressed octahedra. Figure 4.45 A metal-ligand m,—orbital splitting diagram depicting interaction of the metal-atom d NAO and ligand nL NBO to form semi-localized NLMOs of the coordination complex...
Energy of Orbitals - Calculating the Energy Level, Solved ... The s-orbital has the azimuthal quantum number value of 0, the p-orbital has a value of 1, and the d-orbital has two and f-orbital 3. The orbital energy levels of these are varied, s-orbital having the lowest energy level and f-orbital being the highest, summed up as s < p < d < f.
Chem 324 Fall 2009 Quiz #3 KEY NAME splitting of the d orbital energies. 2. Explain why a d ... pt) NOTE: represent your answer as a qualitative energy level diagram.2 pages
Quantum Number Orbital - Definition, Formula, Diagram, Shape Bohr's model hydrogen could not explain the splitting of a single spectral line into a number of closely spaced lines in presence of a magnetic field or the presence Atomic orbitals define the basic building blocks of the quantum orbital diagram or alternatively known as the electron wave mechanics model.
d orbital splitting diagram for metal center and fill it... | Course Hero _ molecular orbital diagram. University of California, San Diego • CHEMISTRY 120A. Fall 2010 CHEMISTRY 120A EXAM 2 Key. Sketch a Molecular Orbital Diagram.
Bonding in Coordination Compounds: Crystal Field Theory | Boundless... Octahedral CFT splitting: Electron diagram for octahedral d shell splitting. Crystal field stabilization is applicable to the transition-metal complexes of orbital: A specification of the energy and probability density of an electron at any point in an atom or molecule. Color in Coordination Compounds.
Transition Metal d-Orbital Splitting Diagrams: An... | Semantic Scholar The presentation of d-orbital splitting diagrams for square planar transition metal complexes in textbooks and educational materials is often inconsistent and therefore confusing for students.
Lecture 31: Color and Magnetism of Coordination Complexes This d-orbital splitting diagram for Oh is really a simplified molecular orbital diagram for the molecule in which we just look at those orbitals that derive from the set of five d orbitals on that metal ion. Because these are the orbitals, -- -- and, together with the electrons that occupy them, they control the...
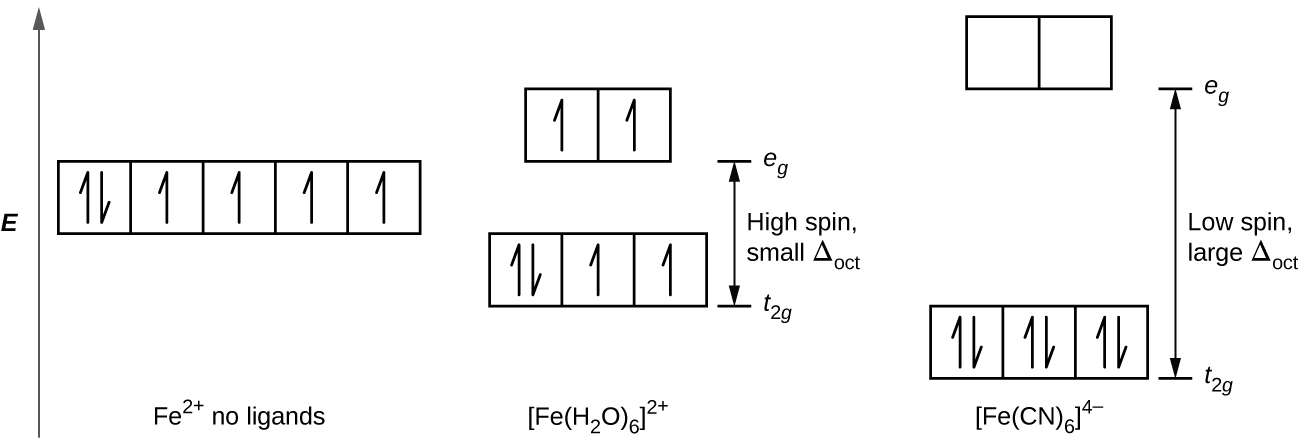
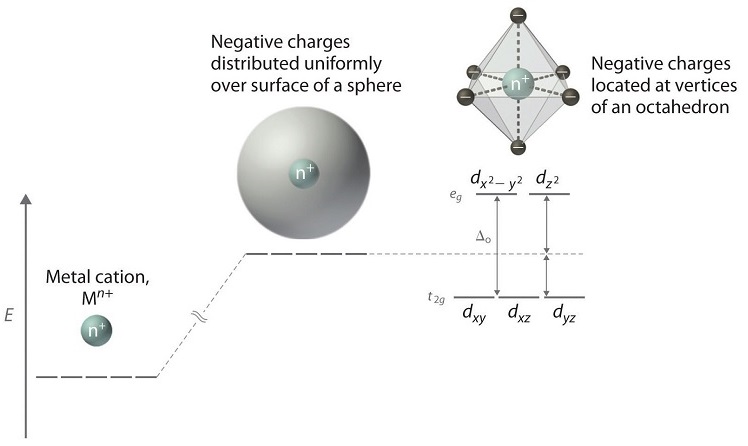
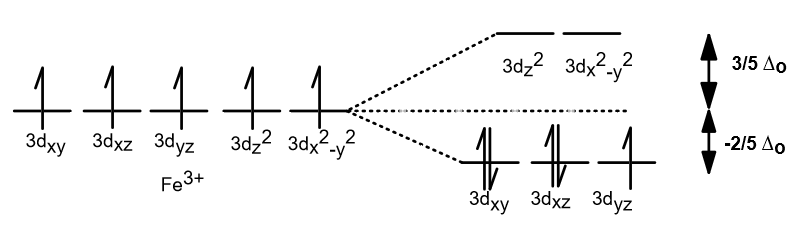
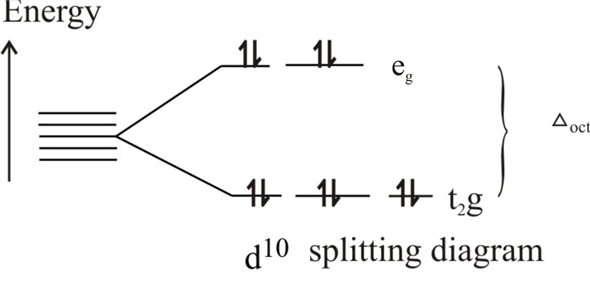
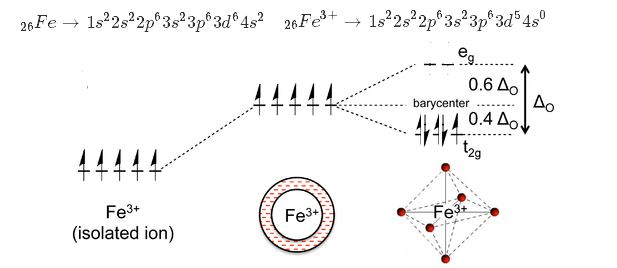
Crystal field theory - Wikipedia If the splitting of the d-orbitals in an octahedral field is Δ oct, the three t 2g orbitals are stabilized relative to the barycenter by 2 / 5 Δ oct, and the e g orbitals are destabilized by 3 / 5 Δ oct.As examples, consider the two d 5 configurations shown further up the page. The low-spin (top) example has five electrons in the t 2g orbitals, so the total CFSE is 5 x 2 / 5 Δ oct = 2Δ oct.
Complex ion d orbital splitting Q : chemhelp Moving down a group you have orbitals size increase and electron density decrease which means ligands can approach closer to the more-extended d orbitals generating higher splitting. Nature of the ligand (spectrochemical series) and also its ability to get closer to the metal centre (hindrance).
PDF D-orbital splitting diagrams Use crystal field theory to gen D-orbital splitting diagrams Use crystal field theory to generate splitting diagrams of the d-orbitals for metal complexes with the following coordination patterns: 1. Octahedral.
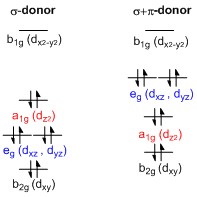
File:D-orbital splitting diagrams of square planar complexes.jpg... English: Representative d-orbital splitting diagrams for square planar complexes featuring σ-donor (left) and σ+π-donor (right) ligands.
PDF Lecture Chapter 23 | Splitting Energy (CFSE) Molecular Orbital Treatment Without going into the group theory considerations of how to set up symmetry adapted atomic orbitals on the metals and the ligands. d - orbital splitting diagrams In the limit of going from ML6 to ML4: (Octahedral to square planar).
D orbital splitting diagram? Fe(CN) 6 4- - HomeworkLib Add Answer of: D orbital splitting diagram? Fe(CN)6 4 Draw a labelled orbital energy level diagram that shows both the splitting of the d-orbitals and their electron occupancy in [Fe(CN)6]. (3 marks) (ii) Fe(III) can also form tetrahedral complexes, most of which are weak field.
PDF Microsoft Word - Ch112_2017_PS6_AK.docx 30th Problem 2 (3 points) The d orbital splitting diagram of Potassium Tetrachloroplatinate(II) has been debated in numerous articles over the years. Absorption spectra collected with polarized light helped elucidating the d-orbital splitting of this complex.
Enhanced charge separation and photocatalytic hydrogen ... Metal halide perovskites are promising candidates as photocatalysts due to their uniquely outstanding photophysical properties; however, the catalytic efficiency is limited by severe charge recombination. Herein, we show that carbonized polymer dots (CPDs) can act as an efficient charge modulator to stabiliz Horizons Community Board Collection: Solar Energy Conversion
SOLVED:Draw the d -orbital splitting diagrams for the octahedral... VIDEO ANSWER: brother Deon, disputing diagrams for the optical components. Irons off each other for me, Zeke. Work indigenous Soon we're struggling with zinc. Zinc at the mid number is turkey. And here's that in da.
Crystal Field Splitting - an overview | ScienceDirect Topics Crystal field d orbital splitting diagrams for common geometries. The above treatment considers the ligands in an octahedral geometry (i.e., with the ligands placed at the centre of the faces of the cube). The square planar case is simply a special case of the octahedral symmetry where two ligands are...
Inorgo D Orbital Splitting Diagrams Flashcards | Quizlet Start studying Inorgo D Orbital Splitting Diagrams. Learn vocabulary, terms and more with flashcards, games and other study tools. Only RUB 193.34/month. Inorgo D Orbital Splitting Diagrams.
How to Do Orbital Diagrams | Sciencing Orbital diagrams give you all of the information you need about the electron configuration and occupied spin states for chemistry or physics, and are easy Dot diagrams are very different to orbital diagrams, but they're still very easy to understand. They consist of the symbol for the element in the...
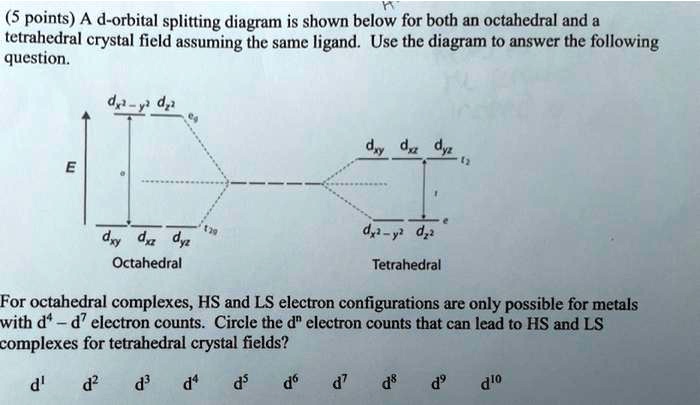
Coordination Chemistry III: Tanabe-Sugano Diagrams and ... As a result, we can use octahedral d10-nT-S diagrams to describe dn tetrahedral complexes. For example, d8looks like d2octahedral, d7 looks like d3, etc. Hole Formalism:since the splitting of the d-orbitals is opposite in tetrahedral and octahedral complexes, tetrahedral configurations with
Answered: PRE-LAB QUESTION After consulting… | bartleby Mar 17, 2022 · PRE-LAB QUESTION After consulting sections 5.7 of your text, draw the complete MO diagram for the diatomic molecule, H2. Next to each of the MOs in your diagram, draw a picture that describes the constructive or destructive interference of atomic wave functions and indicate the location of any nodes.
d-Metal Complexes This theory explains the splitting of the d orbitals to remove their degeneracy, the number of unpaired electrons in transition metal complexes, their color, spectra and magnetic properties. In this case, the dz2 orbital drops even lower in energy, and the molecule has the following orbital splitting diagram.
Crystal Field Theory | d-Orbital Splittings d-Orbital Splittings. CFT focuses on the interaction of the five (n − 1)d orbitals with ligands arranged in a regular array around a transition-metal ion. We can use the d-orbital energy-level diagram in Figure The splitting of the d orbitals because of their interaction with the ligands in a complex has...















/Octahedral_crystal-field_splitting-589932a85f9b5874ee4e3368.png)







![Why is[FeF6]3- ion paramagnetic while [Fe(CN)6]4-ion ...](https://miro.medium.com/max/1240/0*17eRFAsr7bmIIkGX.jpg)
0 Response to "44 d orbital splitting diagram"
Post a Comment