45 molecular orbital diagram for ne2
8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. However, we can predict that the Be2 molecule and the Ne2 molecule would not be stable. We can see this by a consideration of the molecular electron configurations (Table 8.3). PDF Microsoft Word - Handin8s2017ans.docx 1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. Assume the valence 5s-orbitals of Xe are sufficiently Determine the primary MOs that determine the bond order. Compare the general features of your MO diagram to the MO diagram for [F-H-F]...
Molecular Orbital Theory - Chemistry: Atoms First 2e This is the molecular orbital diagram for the homonuclear diatomic showing the molecular orbitals of the valence shell only. However, we can predict that the Be2 molecule and the Ne2 molecule would not be stable. We can see this by a consideration of the molecular electron configurations ((Figure)).
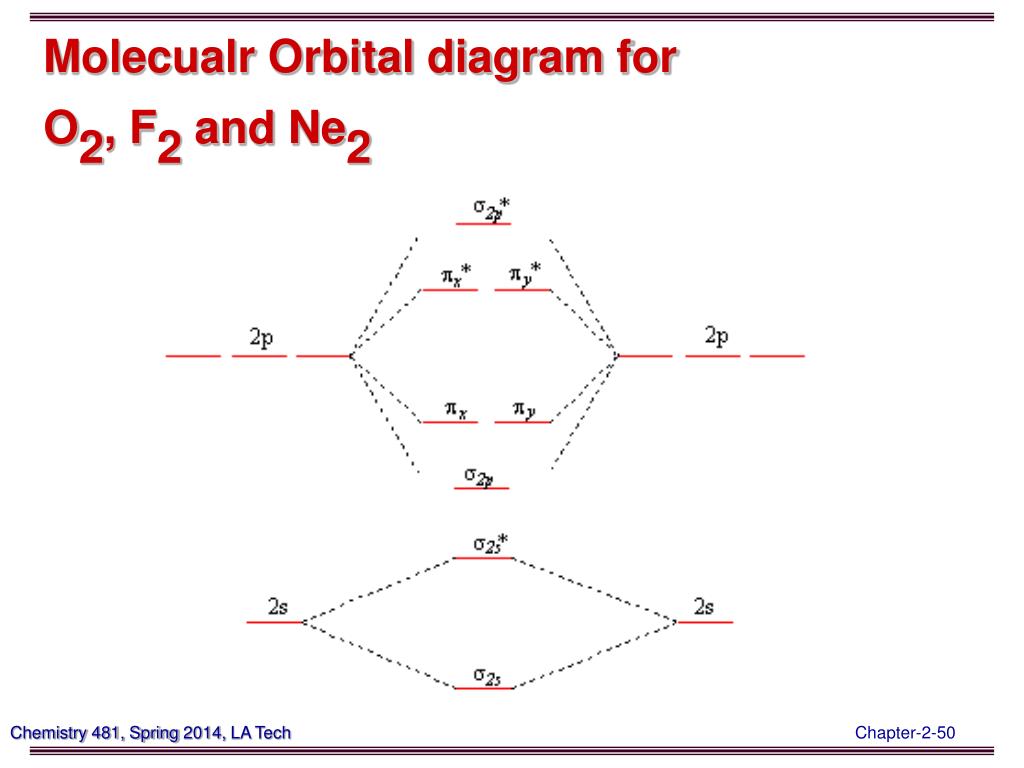
Molecular orbital diagram for ne2
What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2p_(sigma), so that is where the extra electron will be added. The problem provides you with the MO diagram for the #"C"_2# molecule, so all you really have to do here is add an electron to that diagram. 2.7 Molecular Orbital Theory - Inorganic Chemistry for Chemical... Looking at Ne2 molecular orbitals, we see that the order is consistent with the generic diagram shown in the previous section. Figure 2.7.11 - This shows the MO diagrams for each homonuclear diatomic molecule in the second period. The orbital energies decrease across the period as the effective... Why does the molecular orbital diagram for Be2+ consist of... For complete MO diagrams, you use the total number of electrons. The 1s electrons of O2, N2, etc. are used to fill up the sigma(1s) and sigma(1s) Remember, valence electrons are those which do not represent a noble-gas-like-state. the 1s Orbital is full (2 electrons), so the Be2+ configuration is the...
Molecular orbital diagram for ne2. PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams Figure 9-2 Molecular orbital (MO) diagram for the combination of the 1s atomic orbitals on two identical atoms (at the left) to form two MOs. Molecular orbital calculations indicate, however, that for O2, F2, and hypothetical Ne2 molecules, the 2p orbital is lower in energy than the 2p orbitals... Molecular orbital diagram - Infogalactic: the planetary knowledge core A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular. PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... - MO diagrams for Inorganic complexes. • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory. Explain Energy Level Diagram for Molecular Orbitals Thus, for diatomic molecules of second period (Li2 to Ne2), there are two types of energy levels of Mos. For molecules Li2, Be2, B2, C2 and N2 the molecular orbital energy level diagram.
Atomic and Molecular Orbital Diagram for Oxygen/O2 Describe molecular bonding using molecular orbital theory, compare/contrast with VESPR and Lewis theory. Understand how to draw & fill molecular orbital Molecular Diagram of B C N (Vs) O F Ne, how to draw & fill diagrams. Understand how to represent electron densities for sigma, pi bonding... Figure 14: The molecular orbital energy-level diagram for diatomic... The molecular orbital energy- level diagram that results is constructed by putting the molecular orbitals in order of increasing number of internuclear nodal planes, the orbital with no such nodal plane lying at lowest energy and the orbital with nodal planes between all the atoms lying at highest energy. How is the molecular orbital diagram of N2 determined? - Quora Let me explain the molecular orbital diagram of N2 using its diagram. one atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons. How many d orbitals are involved? Why does the Ne2 molecule not exist using molecular orbital theory? What is the molecular orbital diagram for... MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row
PDF Chapter 5 | 5.2.2 Orbital Mixing Molecular orbital theory uses group theory to describe the bonding in molecules; it comple-ments and extends the introductory bonding models in Chapter 3 . In molecular orbital theory the symmetry properties and relative energies of atomic orbitals determine how these orbitals interact to form... Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep The molecular orbital diagram representing this order of energy levels is shown in fig. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if the value is negative or zero, it means that the molecule is unstable. Molecular Orbital Diagrams - Every Science The procedure for working out a molecular orbital of a general diatomic molecule is quite simple. We construct molecular orbitals using the available orbitals on Thus for the elements O to Ne, which we shall consider initially, the effects of this new principle are not observed. The MO diagram for these... PDF Figure 9.32: The molecular orbital energy-level diagram for • The following slide illustrates the relative energies of the molecular orbitals compared to the original atomic orbitals. • Because the energy of the two electrons is lower than the energy of the individual atoms, the molecule is stable. Figure 9.26: (a) The molecular orbital energy-level diagram for the...
PDF Slide 1 "Ne2". Molecular Orbital Energy Level Diagram for a Heteronuclear Diatomic. e.g. for CO - similar to N2 - but with different a.o. energies for C and O, i.e. O > C.
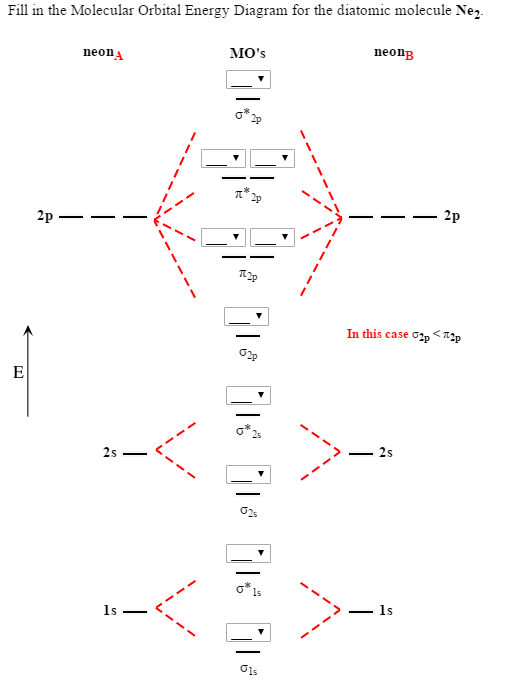
Draw and explain the molecular orbital diagram of Ne2 . On the basis... zumba12. Molecular orbital diagram of. Explanation: Neon atom has 10 electrons and its electronic configuration is . As the bond order value for molecule is zero, it is unstable and cannot exist. The molecular orbital diagram of hypothetical molecule is given in the attachment.
Chapter 9 Molecular Orbitals in Chemical Bonding (Midterm) | Quizlet no two molecular orbitals for any molecule ever have the same energy. which of the following statements concerning the molecular orbital energy level diagrams for first and second period homonuclear diatomic molecules is FALSE. the diagram for O2, F2, and Ne2 molecules has the...
Molecular orbital diagram — Wikipedia Republished // WIKI 2 A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.[1][2][3] A fundamental principle of these theories is that...
Asked for: molecular orbital energy-level diagram, valence electron... A molecular orbital is an allowed spatial distribution of electrons in a molecule that is associated with a particular orbital energy. Use a molecular orbital energy-level diagram to predict the valence-electron configuration and bond order of the H 2 2 − ion. Is this a stable species?
PDF Microsoft PowerPoint - Polyatomic Molecular Orbital Theory... Polyatomic Molecular Orbital Theory. Transformational properties of atomic orbitals. 1.1 -. B2 10 1 2. C2 12 2 0 N2 14 3 0 O2 16 2 2 F2 18 1 0 Ne2 20 0 0. The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure.
Energy level diagram for Molecular orbitals - Chemical Bonding and... Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons. 3) If Nb = Na ,the molecule is again unstable because influence of electrons in the antibonding molecular orbital is greater than the bond influence of...
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
Solved Draw the molecular orbital diagram for Ne2+ and | Chegg.com If 2p orbitals on an atom are all the same energy, why do they form molecular orbitals of different engergies when theu mix?
Molecular Orbital Theory This molecular orbital model can be used to explain why He2 molecules don't exist. Combining a pair of helium atoms with 1s2 electron configurations would The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the...
Molecular Orbital Theory: Energy level diagram for molecular orbitals Molecular orbital theory was put forward by Hund and Mullikan in 1932. This theory is modern This energy diagram for the molecular orbitals is shown in Fig.1 However, experimental evidence MOs is valid for molecules or ions like O2, O (super oxide ion), O22-(peroxide ion), F2 and Ne2 (hypothetical).
Why does the molecular orbital diagram for Be2+ consist of... For complete MO diagrams, you use the total number of electrons. The 1s electrons of O2, N2, etc. are used to fill up the sigma(1s) and sigma(1s) Remember, valence electrons are those which do not represent a noble-gas-like-state. the 1s Orbital is full (2 electrons), so the Be2+ configuration is the...
2.7 Molecular Orbital Theory - Inorganic Chemistry for Chemical... Looking at Ne2 molecular orbitals, we see that the order is consistent with the generic diagram shown in the previous section. Figure 2.7.11 - This shows the MO diagrams for each homonuclear diatomic molecule in the second period. The orbital energies decrease across the period as the effective...
What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2p_(sigma), so that is where the extra electron will be added. The problem provides you with the MO diagram for the #"C"_2# molecule, so all you really have to do here is add an electron to that diagram.
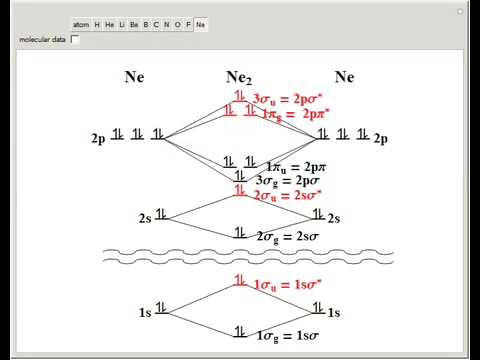
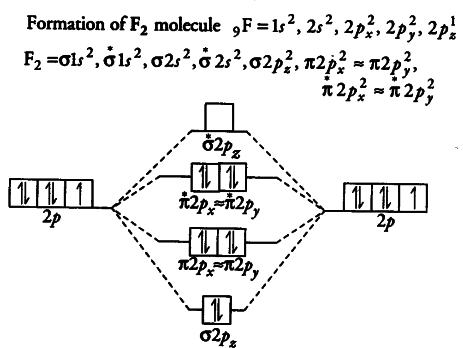



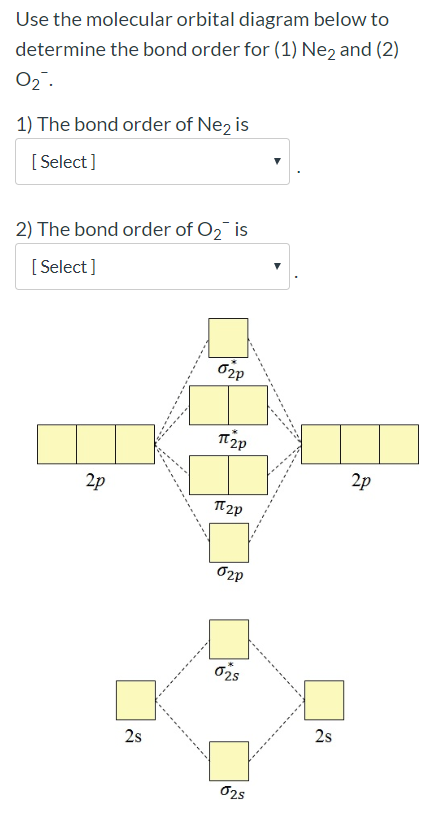
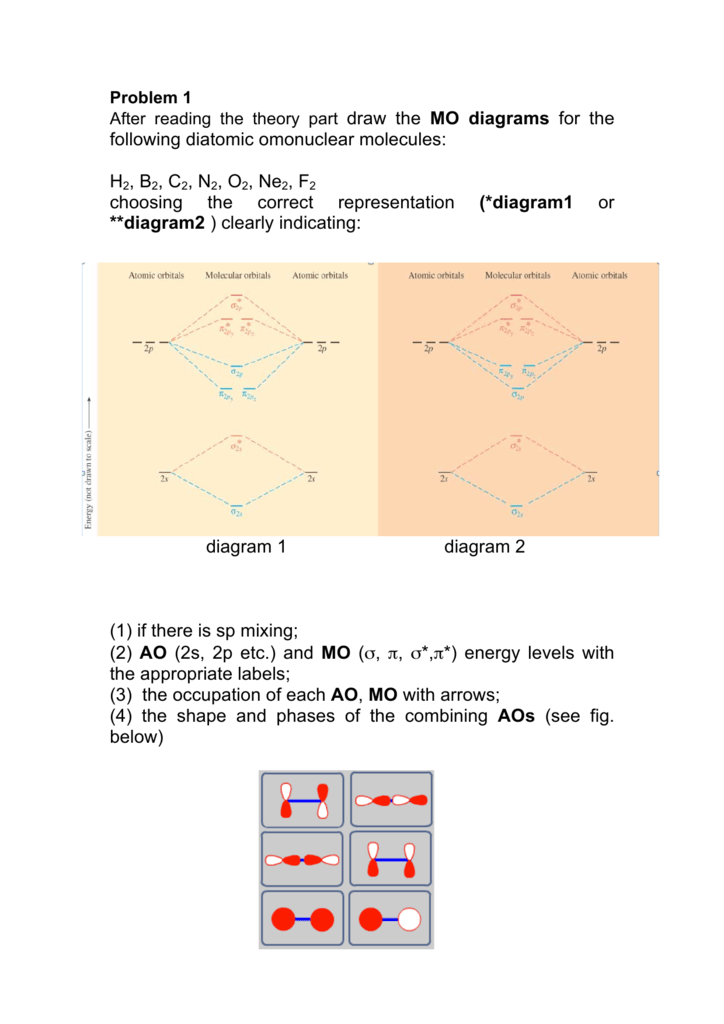

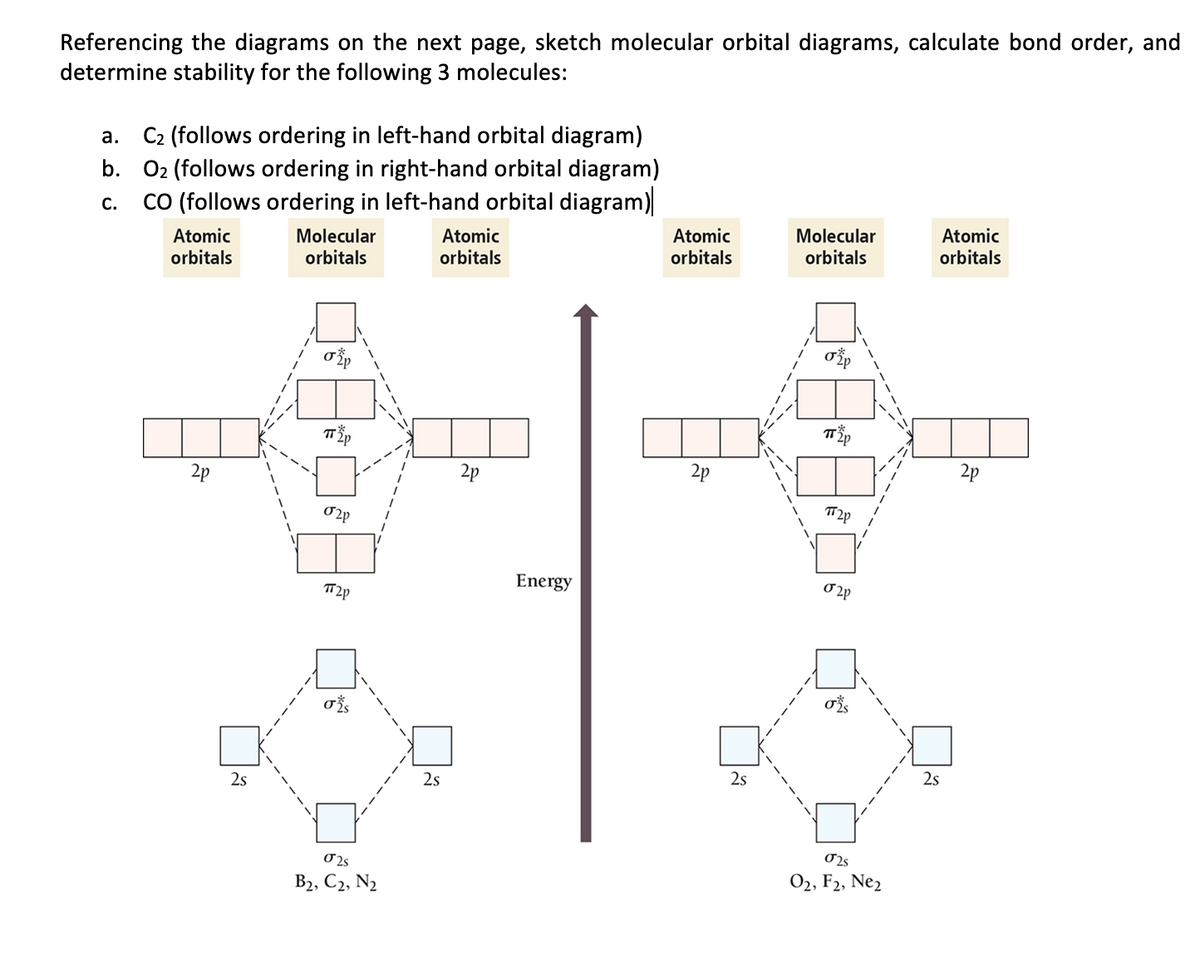
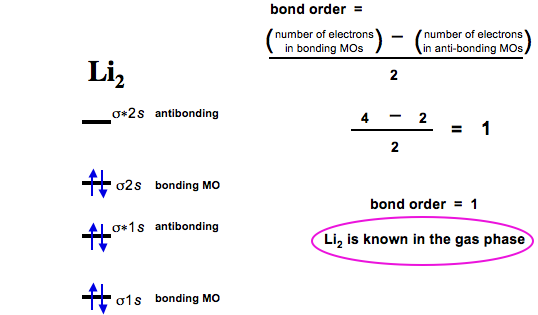


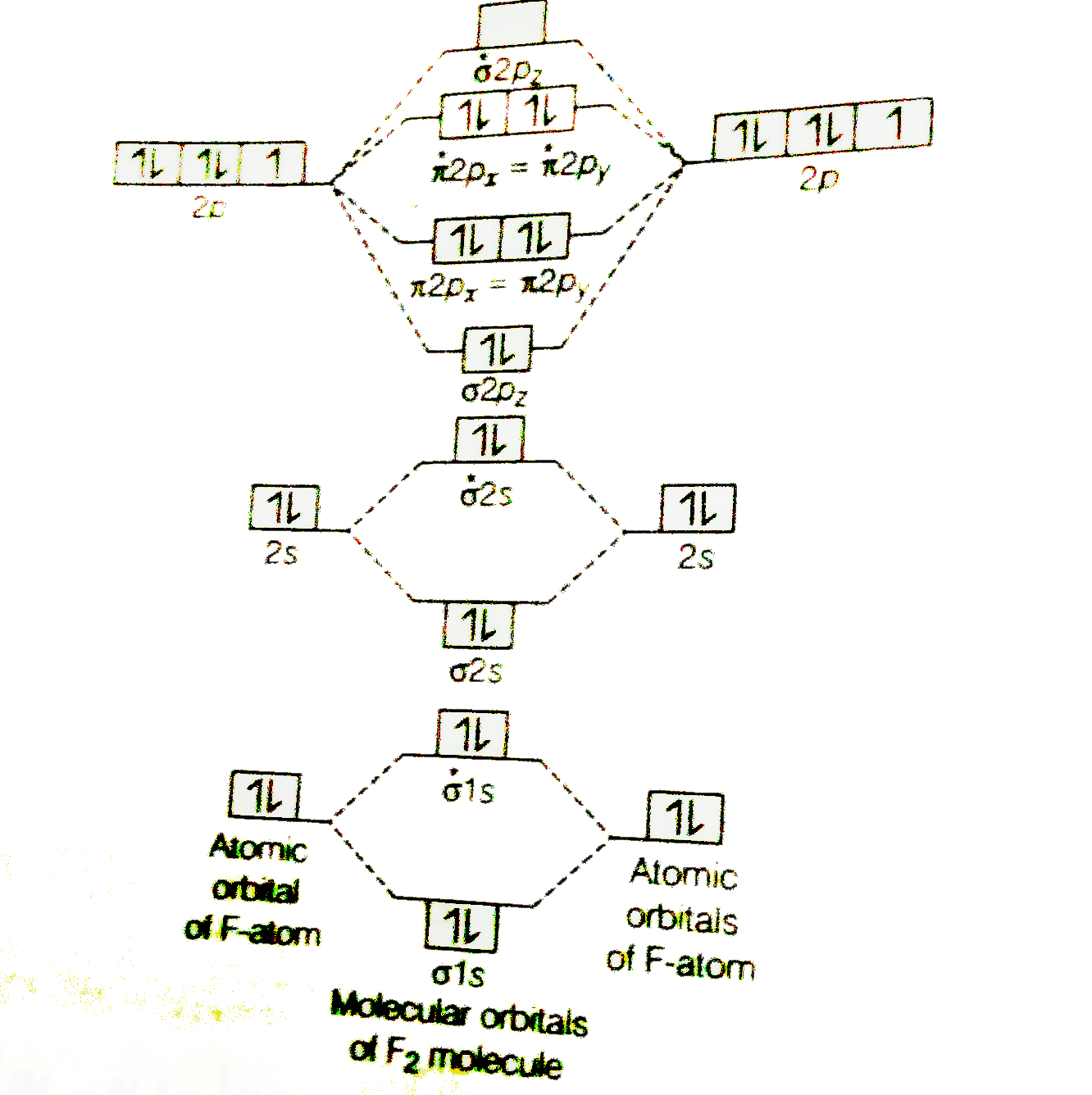

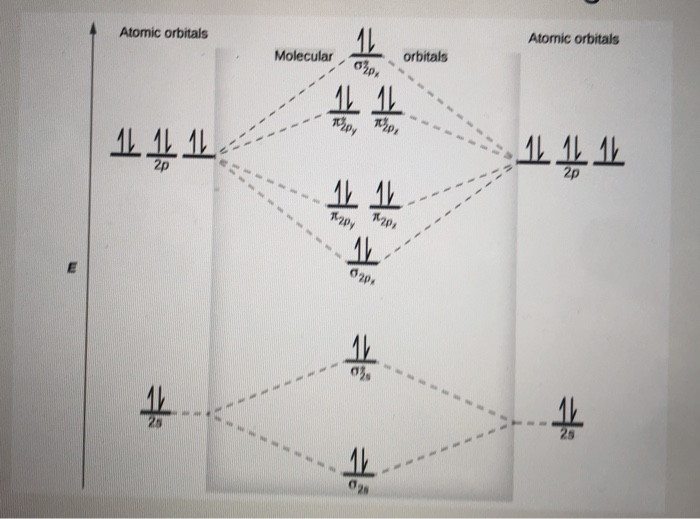







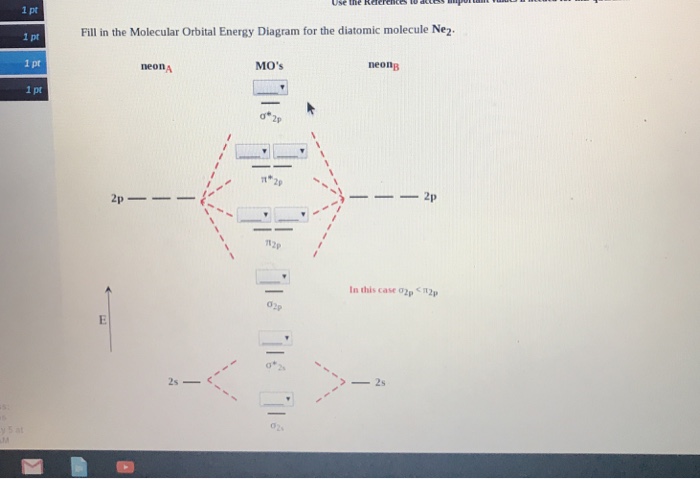

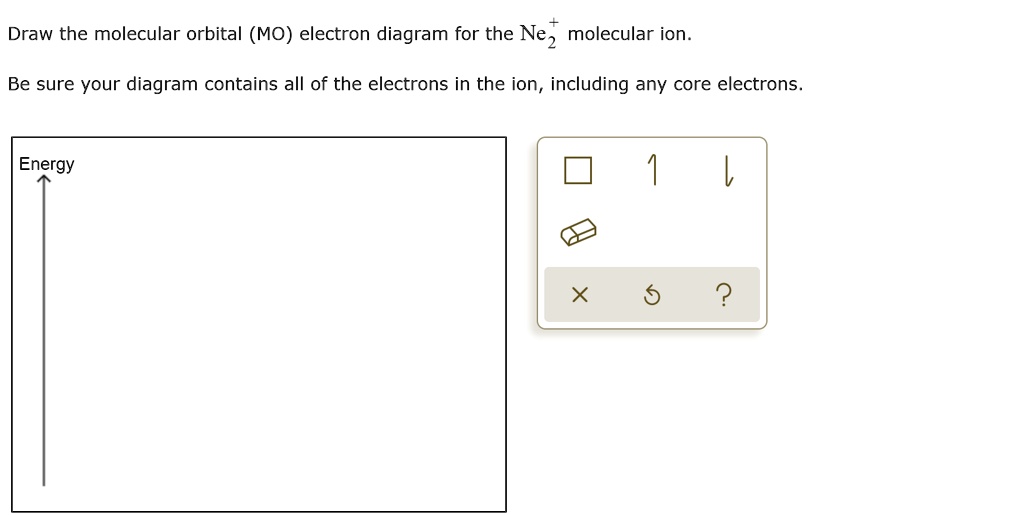
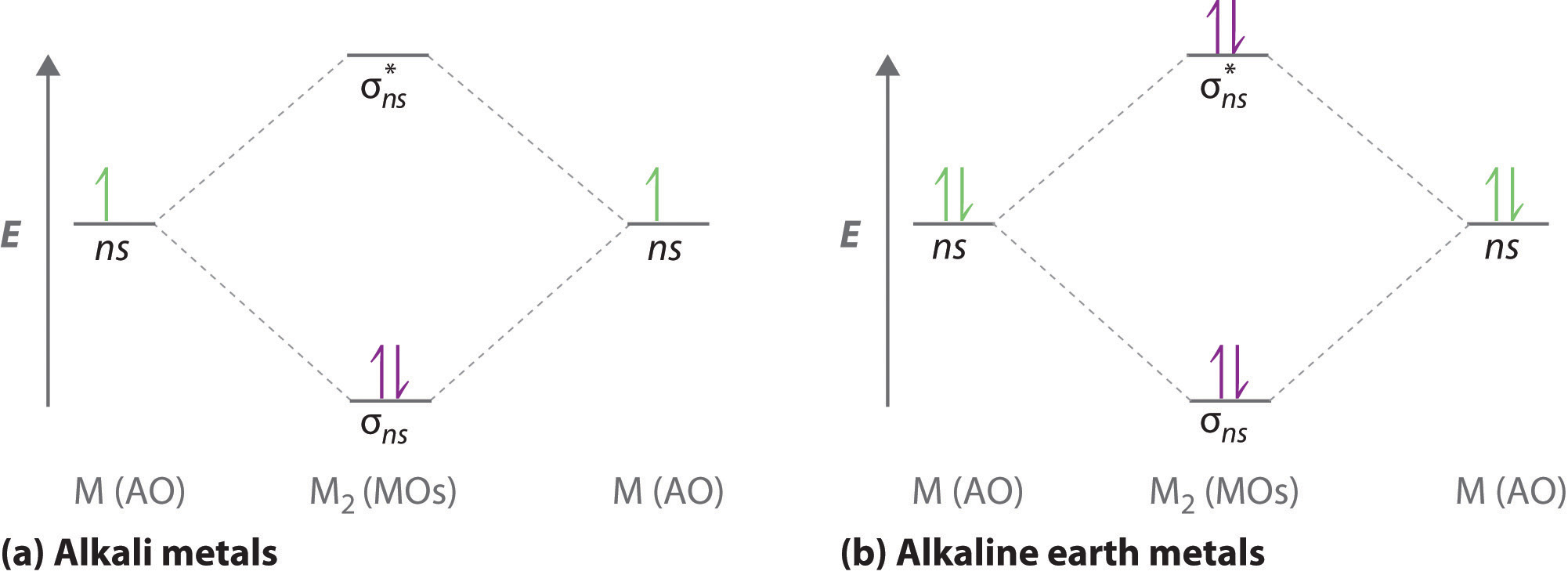



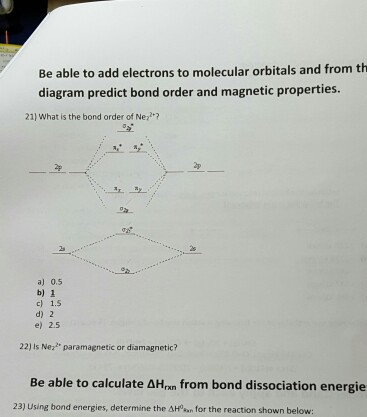
![Expert Verified] using MOT find out the bond order of Ne2 ...](https://hi-static.z-dn.net/files/d9f/f4e9ccc0480e0f453254060b5fef8c9a.jpg)
0 Response to "45 molecular orbital diagram for ne2"
Post a Comment