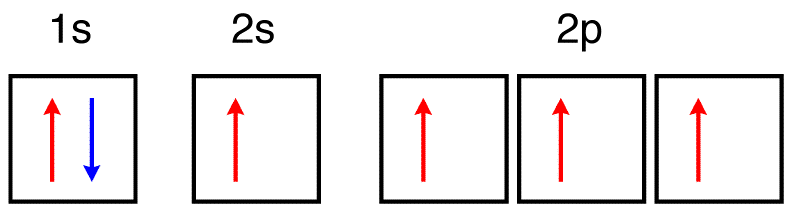
42 the orbital diagram for a ground state oxygen atom is
› articles › s41467/020/16848-8Engineering unsymmetrically coordinated Cu-S1N3 single atom ... Jun 16, 2020 · Engineering unsymmetrically coordinated Cu-S 1 N 3 single atom sites with enhanced oxygen ... at the top site of Cu atom, so the O p orbital could ... ground state energy, zero ... The orbital diagram for a ground-state oxygen atom is ... The orbital diagram for a ground-state oxygen atom is? Orbital diagrams All elements have the same orbitals, arranged in the same order: 1s2s2p3s3p3d4s.... The orbitals fill from the lowest-energy...
the orbital diagram for a ground state oxygen atom is Get the detailed answer: the orbital diagram for a ground state oxygen atom is ... the orbital diagram for a ground state oxygen atom is. Answer +20. Watch. 1. answer. 0. watching. 97. views. For unlimited access to Homework Help, a Homework+ subscription is required.

The orbital diagram for a ground state oxygen atom is
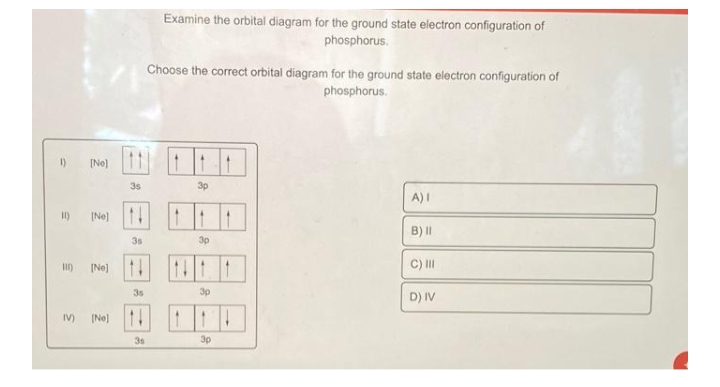
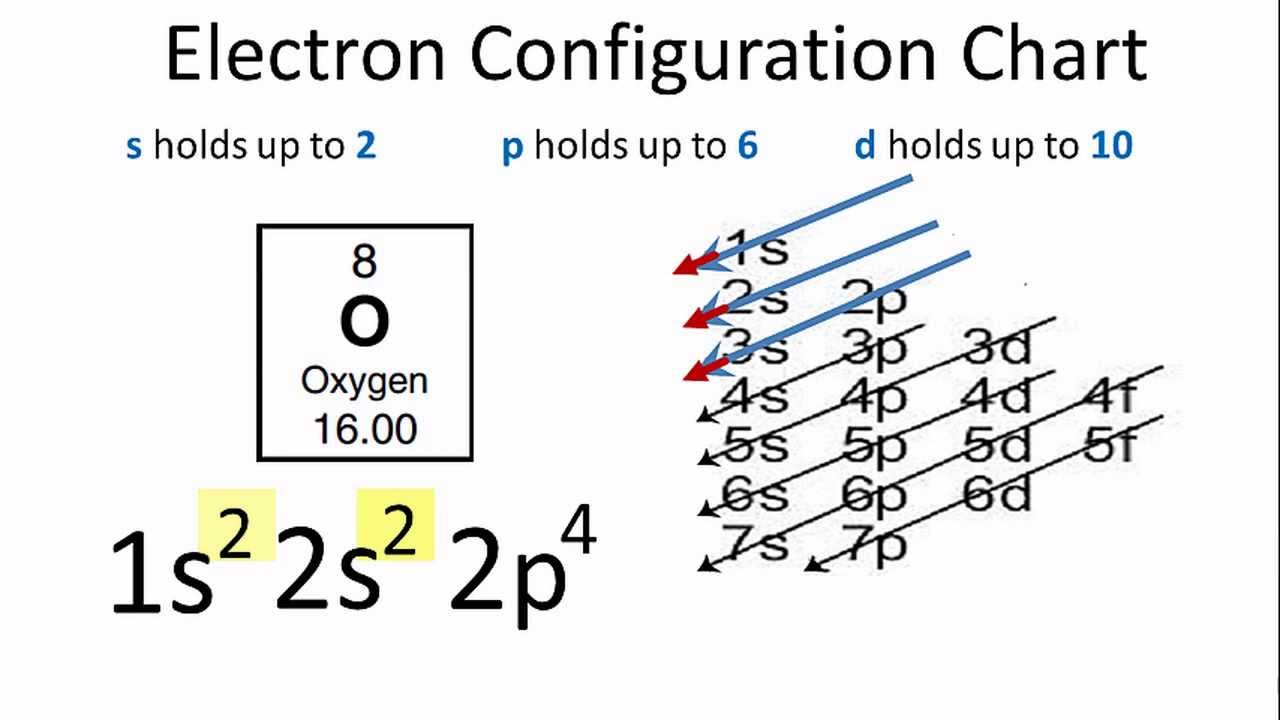
What is the ground state of oxygen atom ... In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be 1s22s22p4. nysedregents.org › Chemistry › 819The University of the State of New York REGENTS HIGH SCHOOL ... 4 An orbital is a region in an atom where there is a high probability of fi nding (1) an alpha particle (3) a neutron (2) an electron (4) a positron 5 Which electron shell in an atom of calcium in the ground state has an electron with the greatest amount of energy? (1) 1 (3) 3 (2) 2 (4) 4 6 As the elements in Period 2 are considered Q9 which of the following electron ... - Course Hero The orbital diagram for a ground-state oxygen atom is Answer: D. Answer : D. Q25. The orbital diagram for a ground-state carbon atom is Answer: D. Answer : D. Q26. Which ground-state atom has an electron configuration described by the following orbital diagram? [Ne] A. phosphorus B. nitrogen C. arsenic D. vanadium.
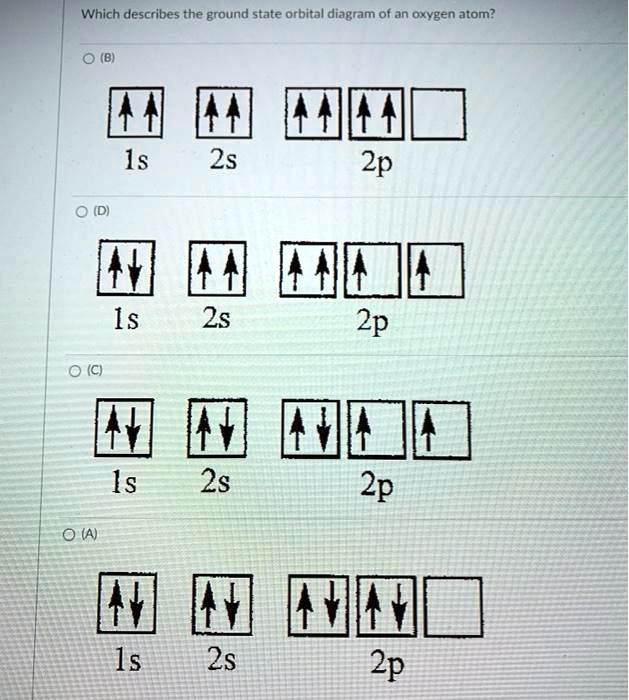
The orbital diagram for a ground state oxygen atom is. techiescientist.com › hcooh-lewis-structureHCOOH Lewis Structure, Molecular Geometry ... - Techiescientist Mar 28, 2022 · As the carbon atom form three sigma bonds with other atoms and hence, one 2s orbital and two 2p orbitals of the carbon atom will mix and form three sp2 hybrid orbitals and one of the p orbitals remains unhybridized, which form a pi bond with the oxygen atom. The orbital diagram of formic acid, which represents the sigma bonds, is shown below. Each neutral atom of beryllium has 4 protons and 4 ... 👍 Correct answer to the question Each neutral atom of beryllium has 4 protons and 4 electrons. Which of the following shows the correct orbital diagram for beryllium in its ground state? - ehomework-helper.com Electron Configuration for Oxygen (O) In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be 1s 2 2s 2 2p 4. Video: Oxygen Electron Configuration Notation Solved The orbital diagram for a ground-state oxygen atom is Chemistry Chemistry questions and answers The orbital diagram for a ground-state oxygen atom is 1s 2s C. D.↑↓ ↑↓ ↑↓↑ ↑ Question: The orbital diagram for a ground-state oxygen atom is 1s 2s C. D.↑↓ ↑↓ ↑↓↑ ↑ This problem has been solved! See the answer Show transcribed image text Expert Answer 100% (10 ratings)
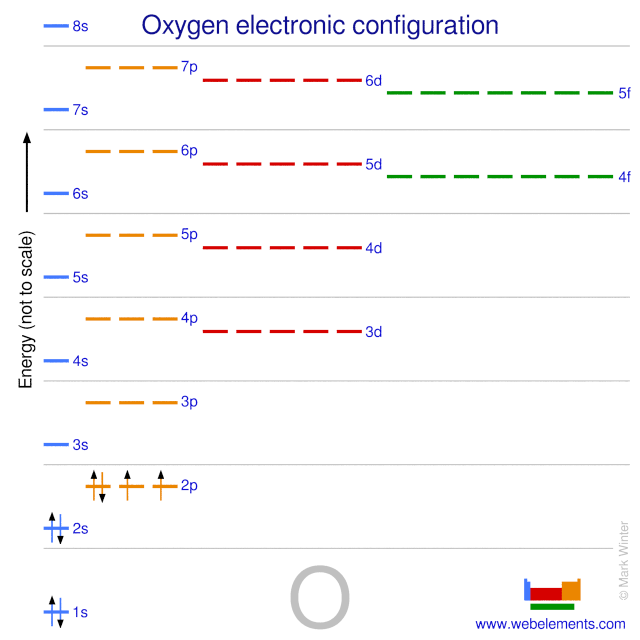
(Get Answer) - 25. Consider the orbital diagram for oxygen ... Explain how the orbital diagram for sodium (Na) confirms that the 3s sublevel is lower in energy than the 3p sublevel. 28. The lowest potential energy arrangement of electrons in an atom is called the ground state. Ground state electron configurations can be predicted by a strict set of rules known as the Aufbau principle ("aufbau"means filling ... orbital diagram of oxygen - menuswitch.com Atomic Orbital Diagram for Oxygen: Atomic orbital diagram for oxygen: The electronic configuration of oxygen (Z=8) in the ground state is 1s 2 2s 2 2p 4. Each oxygen atom has 8 electrons; hence in O22 is as follows molecule there are 16 electrons. therefore, oxygen molecule is a paramagnetic in nature. Hund's Rule and Orbital Filling Diagrams | Chemistry for ... Orbital filling diagram for carbon. Oxygen has four 2 p electrons. After each 2 p orbital has one electron in it, the fourth electron can be placed in the first 2 p orbital with a spin opposite that of the other electron in that orbital. Figure 4. Orbital filling diagram for oxygen. Summary What is the orbital diagram for a ground-state nitrogen ... atom has 7 electrons. The s orbitals can only hold 2 electrons, and the p orbitals can hold up to 6 electrons. The 1s orbital is filled first, leaving five electrons, then the 2s orbital is filled,...
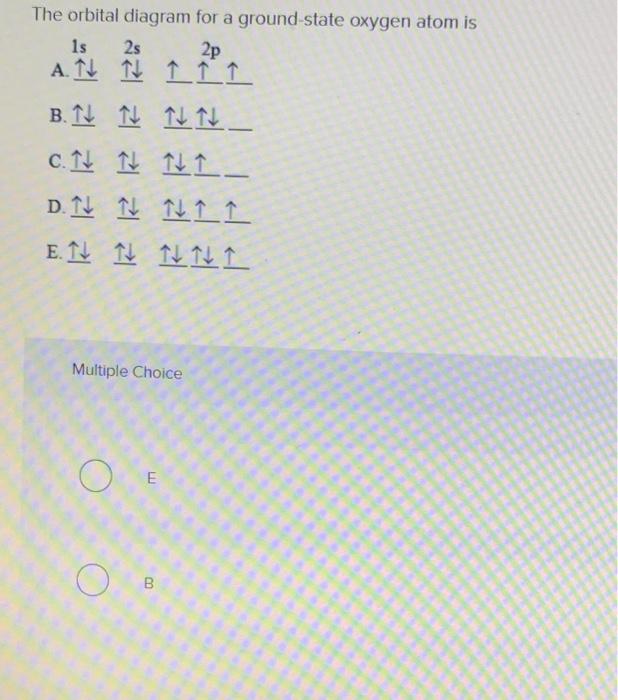
The orbital diagram for a ground-state oxygen atom is Js 2s A ... Answer. Which of the following is the correct orbital diagram for the ground-state electron configuration of molybdenum? Explain what is wrong with each of ...4 answers · Top answer: hi there for this problem. We are asked to identify the correct orbital diagram Formal Injun, ... Chapter 2 Flashcards | Quizlet 5. 1 The orbital diagram for a ground state nitrogen (7) atom is: A. 2 The orbital diagram for a ground state oxygen (8) atom is: D. 3 The orbital diagram for a ground state carbon (6) atom is: D. 4 Which ground state atom has an electron configuration described by the following orbital diagram? Phosphorus. valenceelectrons.com › oxygen-electron-configurationOxygen(O) electron configuration and orbital diagram Orbital Diagram for Oxygen (O) Oxide ion(O 2–) electron configuration. Ground state electron configuration of oxygen is 1s 2 2s 2 2p x 2 2p y 1 2p z 1. This electron configuration shows that the last shell of oxygen has six electrons. In this case, the valence electrons of oxygen are six. The elements that have 5, 6, or 7 electrons in the ... Carbon(C) electron configuration and orbital diagram Carbon (C) excited state electron configuration and orbital diagram. Then correct electron configuration of carbon in ground state will be 1s 2 2s 2 2p x1 2p y1. Therefore, the valency of carbon is 2. Also, the valency of an element is determined by electron configuration in the excited state.
How do yo write the orbital diagram for oxygen? | Socratic 15 Sept 2016 — The electron configuration for oxygen is: 1s^2 2s^2 2p^4 This video will walk you through the step of writing orbital diagram.1 answer · Explanation: The electron configuration for oxygen is: 1s22s22p4 This video will ...
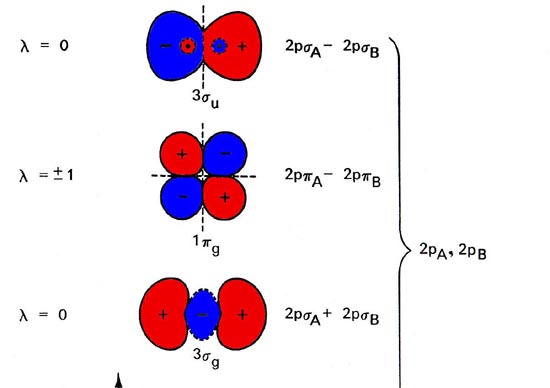
PDF Energetic and chemical reactivity of atomic and molecular ... is interesting to examine the energy diagram of the oxygen atom because similarities with the energy diagram of molecular oxygen can also be used to explain the reactivity of molecular oxygen. ... Therefore, 3P ground state oxygen reacts ... However, the first excited state of atomic oxygen (1D) has one 2p empty orbital.
What is the orbital diagram for carbon? - MSI In the ground state, the highest occupied molecular orbital (HOMO) is the carbon-carbon π bonding orbital; the lowest unoccupied molecular orbital (LUMO) is the carbon-carbon π antibonding orbital. What are the states of carbon? Because it is stable, it can be found both by itself and in many naturally occurring compounds.
Chapter 7: Quantum Theory and the Electronic Structure of ... The orbital diagram for a ground-state nitrogen atom is 13. The orbital diagram for a ground-state oxygen atom is 14. The orbital diagram for a ground state carbon atom is 15. Which ground-state atom has an electron configuration described by the following orbital diagram?
Engineering unsymmetrically coordinated Cu-S1N3 single ... 16.06.2020 · Engineering the coordination environment of single atom catalysts offers to opportunity to optimize electrocatalytic activity. In this work, the authors prepare an unsymmetrical Cu-S1N3 single ...
Carbon Orbital diagram, Electron configuration, and ... The orbital diagram for Carbon is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Carbon orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest two electrons in 2p orbital. Orbital diagram for a ground-state electron configuration of Carbon atom is shown below-.
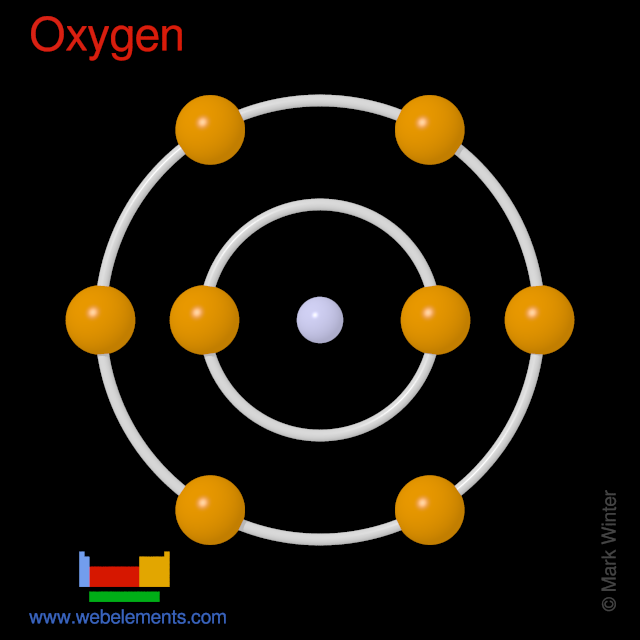
Oxygen(O) electron configuration and orbital diagram Orbital Diagram for Oxygen (O) Oxide ion(O 2–) electron configuration. Ground state electron configuration of oxygen is 1s 2 2s 2 2p x 2 2p y 1 2p z 1. This electron configuration shows that the last shell of oxygen has six electrons. In this case, the valence electrons of oxygen are six. The elements that have 5, 6, or 7 electrons in the ...
en.wikipedia.org › wiki › Orbital_hybridisationOrbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.
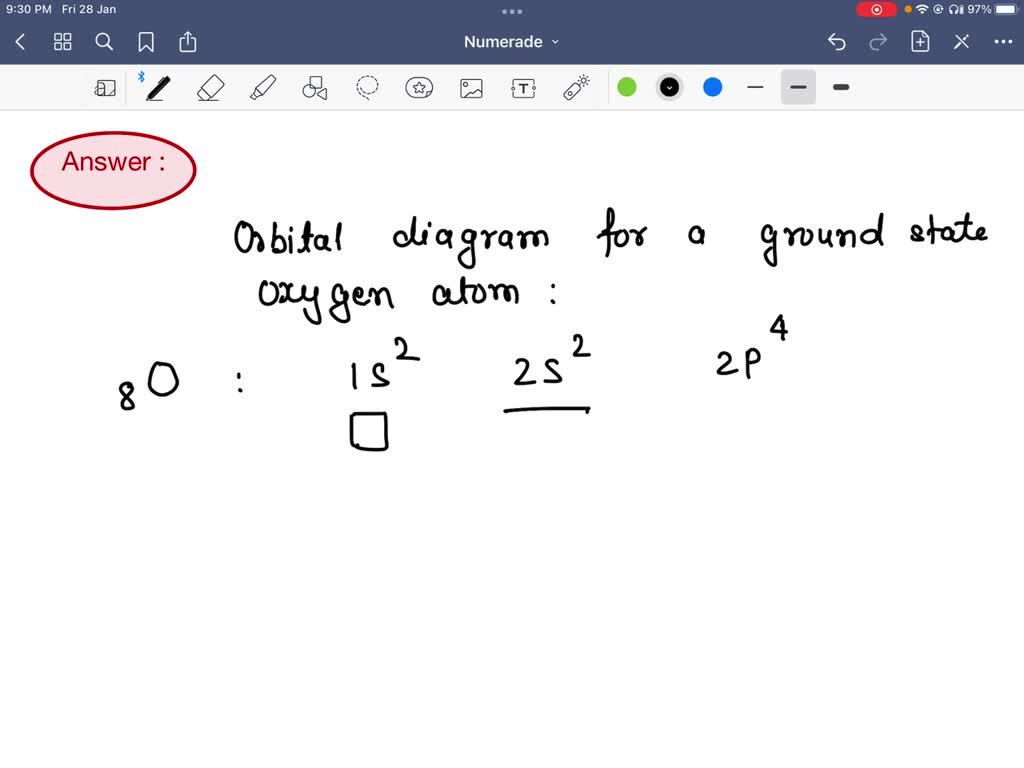
the orbital diagram for a ground state oxygen atom is 1s 2s a _ 1 2_ b_ 1 11 _ c1 1 1l d_ 1 1l_ e_ 1 mill 0 a 0 95222
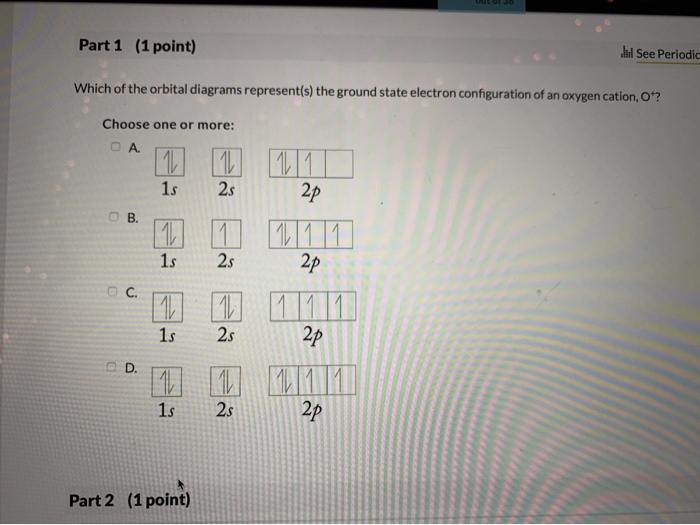
The orbital diagram for a ground-state oxygen atom is 1s B ... consider the o; molecule (a) indicate the ground state orbital diagram, the ground state electron configuration, the bond order and whether o2 in the ground state is diamagnetic or paramagnetic. (8 points) (b) a lewis structure obeying the octet rule can be drawn for o, as follows does the above lewis structure represent a paramagnetic state or a …
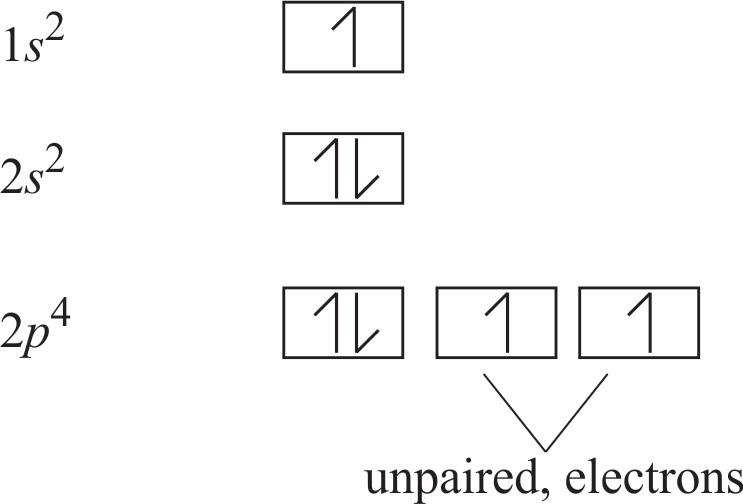
Oxygen Orbital diagram, Electron configuration, and ... The orbital diagram for Oxygen is drawn with 3 orbitals. The orbitals are 1s, 2s, and 2p. The Oxygen orbital diagram contains 2 electrons in 1s orbital, 2 electrons in 2s orbital, and the rest four electrons in 2p orbital. Orbital diagram for a ground-state electron configuration of Oxygen atom is shown below-
Quantum Number Questions and Answers | Study.com Quantum Number Questions and Answers. Get help with your Quantum number homework. Access the answers to hundreds of Quantum number questions that are explained in a way that's easy for you to ...
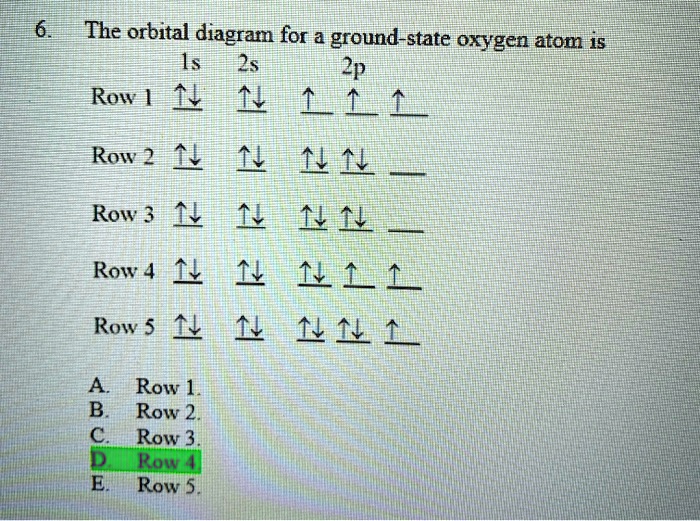
Chapter 7: Quantum Theory and the Electronic Structure of ... 41. The orbital diagram for a ground-state oxygen atom is. Ans: D. Category: Medium Section: 7.8. 42. The orbital diagram for a ground state carbon atom is. Ans: D. Category: Medium Section: 7.8. 43. Which ground-state atom has an electron configuration described by the following orbital diagram?
P a g e 7 15 The orbital diagram for a ground state ... P a g e 7 15 The orbital diagram for a ground state nitrogen atom is 1s 2 2s 2 from PHYS 240 at San Francisco State University
› vinayDesai12 › basic-atomicBasic Atomic structure - SlideShare Dec 06, 2016 · Followed by orbital’s called L-shell, M-shell and N-shell. The maximum no. of electrons in an orbital is given by the formula 2n2. Eg: 1) Hydrogen atom has 1 electron in K-shell 2)Helium atom has 2 electrons in K-shell 3)Oxygen atom has 8 electrons (2 in K-shell, 6 in L-shell) 25.
Ch 7/8 quiz Flashcards | Quizlet The orbital diagram for a ground-state oxygen atom is 1s (up down) 2s (up down) 2p (up down, up, up) Which of the following is the electron configuration of an excited state of an oxygen atom? 1s^2 2s^2 2p^3 3s^1 Select True or False: An electron gains energy in the transition from a 6s subshell to a 5d subshell. true
The orbital diagram for a ground state oxygen atom is ls ... The Orbital Diagram For A Ground-State Nitrogen Atom Is 1s 2s 2p A. It It Î Î Î B.T Ft TuI_ C. Ît Î Î Î D. Ît Îţ Îi Î Î D A B Posted 12 days ago
The orbital diagram for a ground-state oxygen atom is Recent Posts. The blood vessel that supplies blood to the brain from the anterior direction is the . Determine the change in enthalpy for the following reaction from the enthalpies of formation for the reactants and products.NH3 , 46 kJ/mol; NO2 , +33 kJ/mol; H2 O, 286 kJ/mol)
Solved 10) The orbital diagram for a ground-state oxygen ... Question: 10) The orbital diagram for a ground-state oxygen atom is 1s 2s 2p D.I I L11 A) A B) B C) C D) D E) E 11) Which of the following compounds will exhibit hydrogen bonding? A) SiH4 B) PH3 C) H2S D) HF E) CH4 12) Liquid oxygen boils at -182.9°C. Express the boiling point of liquid oxygen in °F.
PDF CHEM1101 2014-J-6 June 2014 A schematic representation of ... e) The oxygen atom in the reaction in part d) is formed in its ground electronic state. What is the ground state electronic configuration for O? Marks 5 1s2 2s2 2p4 Draw an atomic orbital energy level diagram for the ground state O atom. Name the orbitals and show all electrons.
Orbital Diagram For Nitrogen (N) | Nitrogen Electron ... Ground State Electron Configuration For Nitrogen When we talk about the electronic configuration, then the ground state Nitrogen Electron Configuration is written as 1s22s22p3. Below you can get the full image representation which will help you to understand the topic more easily.
Sodium(Na) electron configuration and orbital diagram The 2p orbital is now full. Then next an electron will enter the 3s orbital in the clockwise direction. This is clearly shown in the figure of the orbital diagram of sodium. Sodium ion(Na +) electron configuration. Ground state electron configuration of sodium(Na) is 1s 2 2s 2 2p 6 3s 1. The elements that have 1, 2, or 3 electrons in the last ...
Q9 which of the following electron ... - Course Hero The orbital diagram for a ground-state oxygen atom is Answer: D. Answer : D. Q25. The orbital diagram for a ground-state carbon atom is Answer: D. Answer : D. Q26. Which ground-state atom has an electron configuration described by the following orbital diagram? [Ne] A. phosphorus B. nitrogen C. arsenic D. vanadium.
nysedregents.org › Chemistry › 819The University of the State of New York REGENTS HIGH SCHOOL ... 4 An orbital is a region in an atom where there is a high probability of fi nding (1) an alpha particle (3) a neutron (2) an electron (4) a positron 5 Which electron shell in an atom of calcium in the ground state has an electron with the greatest amount of energy? (1) 1 (3) 3 (2) 2 (4) 4 6 As the elements in Period 2 are considered
What is the ground state of oxygen atom ... In writing the electron configuration for oxygen the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for O go in the 2s orbital. The remaining four electrons will go in the 2p orbital. Therefore the O electron configuration will be 1s22s22p4.
























0 Response to "42 the orbital diagram for a ground state oxygen atom is"
Post a Comment