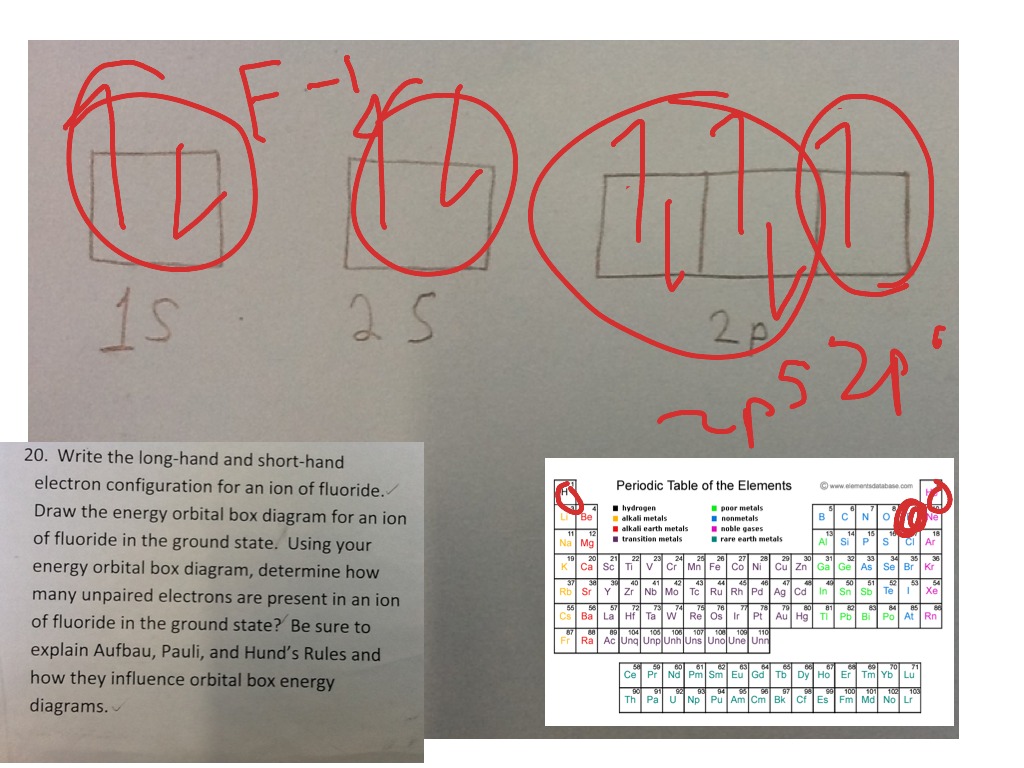
42 orbital diagram of f- ion
After the 4s is full we put the remaining four electrons in the 3d orbital and end with 3d4. Therefore the expected electron configuration for Chromium will be 1s 2 2s 2 2p 6 3s 2 3p 4 4s 2 3d 9. Note that when writing the electron configuration for an atom like Cr, the 3d is usually written before the 4s. Construct the orbital diagram of the f- ion.. How many electrons does a f ion. Construct the orbital diagram of each atom or ion. Write the corresponding electron configuration for. Construct the orbital diagram of the f ion. This problem has been solved. With resolution 3361px x 2298px. Answer to construct the orbital diagram of the f ion.
orbital energy-level diagram for the H2-ion. Bond order = ½ (2 - 1) = 0.5 Electron Configurations of Diatomic Molecules of the Second Period 1. Homonuclear diatomic molecules such as Li 2 utilize only F orbitals. For filled K shell bonding and antibonding orbitals use KK designation. 2.Be2 = KK(F2s)2(F2s*)2 Bond order = 1/2 (2-2) = 0 So Be2 ...
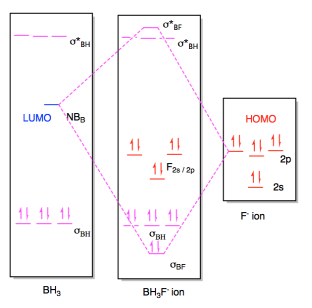
Orbital diagram of f- ion
Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ... We now shift to the 4s orbital where we place the remaining two electrons. Therefore the Calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Video: Calcium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an ... The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ...
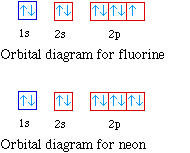
Orbital diagram of f- ion. This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n... Transition Fe3+ ions and draw the orbital box diagrams for both ions. Using this. There for 1s2 2s2 2p6 3s2 3p6 3d5 is the electronic configration for Fe3+. half of electrons (there must be one electron in each orbital, and d has 5 orbitals). That's for filling up orbitals for ground state atoms. Construct the orbital Diagram Of the F– Ion. construct construct the orbital diagram the f– ion chem 120a november 8 2005 fall 2004 8 00 – 9 20 am exam ii name prepare a molecular orbital energy level diagram for no construct a solved construct the orbital diagram the f ion a ne answer to construct the orbital diagram of the f ion a neutral fluorine atom has 9 electrons how many ... Construct the orbital diagram of the f ion. The orbital diagram for bromine is 2 8 18 7. 1s2 2s2 2p5 now the f anion is formed when 1 electron is added to a neutral fluorine atom. What is the max number of electrons that can occupy a box in an orbital filling diagram at any energy level. The remaining five electrons will go in the 2p orbital.
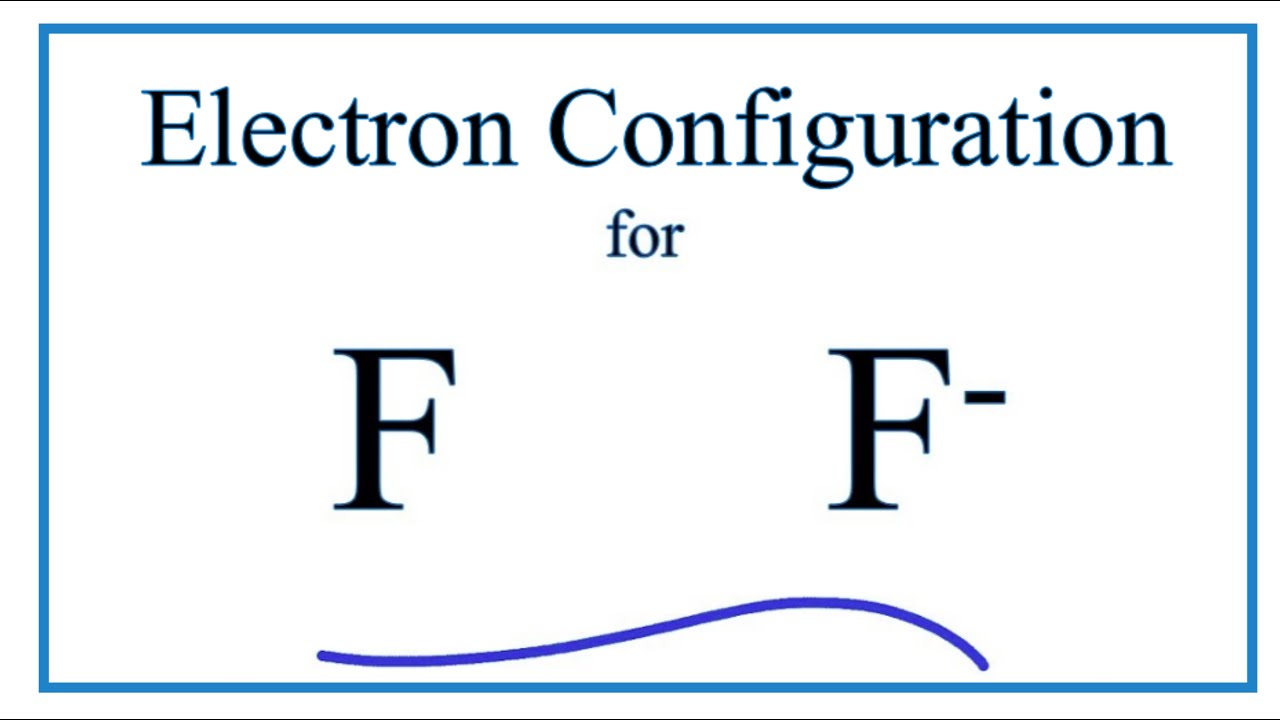
Ca electron occupancy The orbital notation of calcium (Ca) is 1s2 2s2 2p6 3s2 3p6 4s2 Or short-hand: [Ar] 4s2. The orbital filling diagram of lithium. The electron configuration of lithium is 1s²2s¹. This means that there are two electrons in the 1s orbital, and one electron in the higher energy 2s orbital. When we write the configuration we ... When two fluorine atoms bond, the sigma(2p) bonding molecular orbitals are lower in energy than the pi(2p) bonding orbitals.F2(2+) has a bond order of 2, so ... In this video we will write the electron configuration for F-, the Fluoride ion. We’ll also look at why Fluorine forms a 1- ion and how the electron configur... Enter the orbital diagram for the ion Au+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove one electron from 5s1 ANSWER: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10.
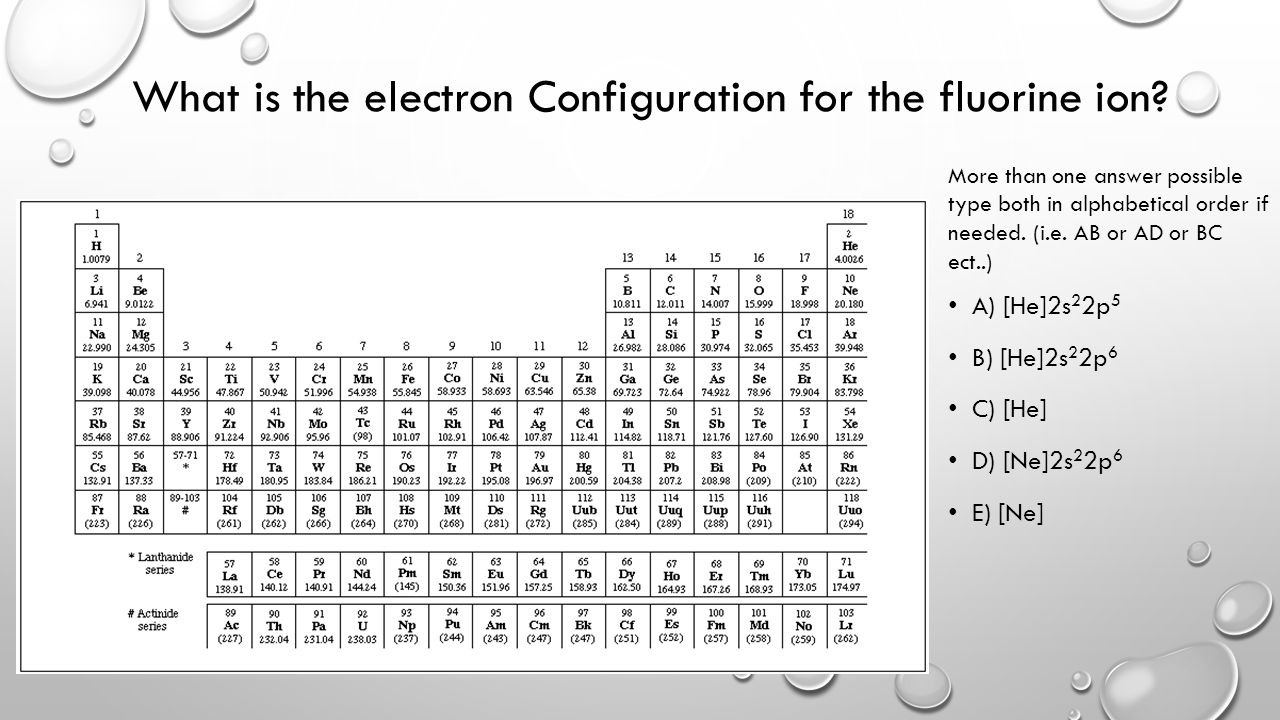
Question: Construct The Orbital Diagram Of The F^- Ion. A Neutral Fluorine Atom Has 9 Electrons. How Many Electrons Does A F^- Ion Have? This problem has been solved! See the answer. Show transcribed image text. Videos. Step-by-step answer 05:59 0 0. Expert Answer 100% (22 ratings) ... Construct the orbital diagram of each atom or ion. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. The 24 electrons of a. Ion electron confugurations. A neutral fluorine atom has 9 electrons. Show transcribed image text construct the orbital diagram of the f ion. "F"^(-): 1s^2 2s^2 2p^6 A good starting point for when you must find the electron configuration of an ion is the electron configuration of the neutral atom. In your case, you must find the electron configuration of the fluoride anion, "F"^(-), so start by writing the electron configuration of a neutral fluorine atom, "F". Fluorine is located in period 2, group 17 of the periodic table and has ... Click within the orbital to add electrons. Jul 21, · Write orbital diagram for Au+ Determine if the ion is diamagnetic or paramagnetic. please helpStatus: Resolved.what is the orbital diagram for Au+, how do you fit the f orbitals in?what is the orbital diagram for Au , how do you fit the f orbitals in?
FREE Expert Solution. We are asked to construct the orbital diagram of the F - ion. F → 9 electrons. Negative charge adds 1 electron more. F - → 10 electrons. 97% (333 ratings)
Fluorine (F) Electron Configuration with Full Orbital Diagram. Fluorine electron configuration is 1s 2 2s 2 2p 5. The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine (F) and the orbital diagram is the main topic of this article.
Orbital diagram of f ion. The following is the diagram for the neutral oxygen. Orbital box diagrams hunds rule pauli exclusion orbital box diagrams are the electron configuration problems where you have to draw each electron out as a little arrow inside a box. The electron configuration for chromium is.
Construct the orbital diagram of the {eq}F^- {/eq} ion. Orbital Diagram: Orbital diagram shows how electrons are distributed in various kinds of shells in the increasing order for a particular ion ...
Feb 23, 2018 · Is this the correct atomic orbital diagram for a calcium 2 ion. Orbital diagram of f ion. The remaining five electrons will go in the 2p orbital. The following is the diagram for the neutral oxygen. Electron configurations orbital diagrams. An orbital diagram naturally leads to the writing of an electron configuration. Ion electron confugurations.
Electron configurations orbital diagrams. 1s22s22p63s23p64s23d4 the orbital diagram above is formatted in such a manner as to place the various orbital types at different energy levels. So the positive ion has a higher bond order by 12 than neutral matho2math.
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H–F nb σ σ* Energy H –13.6 eV 1s F –18.6 eV –40.2 eV 2s 2p So H–F has one σ bond and three lone electron pairs on fluorine
Ti ti2 ti4 construct the orbital diagram of each atom or ion. In this case titanium ti is located in period 4 group 4 of the periodic table and has an atomic number of 22. Write the corresponding electron configuration for. Construct the orbital diagram of the f ion. Draw the orbital diagram for ion ca 2. Get more help from chegg.
Answer (1 of 4): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
This problem has been solved! See the answer. See the answer See the answer done loading. Construct the orbital diagram of the F– ion. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (22 ratings)
Orbital diagram of f ion. It is preferable to have half filled orbitals than incompletely filled orbitals. In writing the electron configuration for fluorine the first two electrons will go in the 1s orbital. The following is the diagram for the neutral oxygen. Fluorine is the ninth element with a total of 9 electrons.
The first number is the principal quantum number (n) and the letter represents the value of l (angular momentum quantum number; 1 = s, 2 = p, 3 = d and 4 = f) for the orbital, and the superscript number tells you how many electrons are in that orbital. Orbital diagrams use the same basic format, but instead of numbers for the electrons, they ...
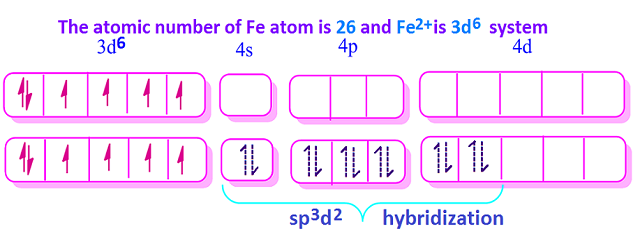
Geometry And Magnetic Properties Of Fe H2o 6 2 Ion By Kakali Ghosh Teacher Blogger M Sc Chemistry Medium
We now shift to the 4s orbital where we place the remaining two electrons. Therefore the Calcium electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2. Video: Calcium Electron Configuration Notation The configuration notation provides an easy way for scientists to write and communicate how electrons are arranged around the nucleus of an ...
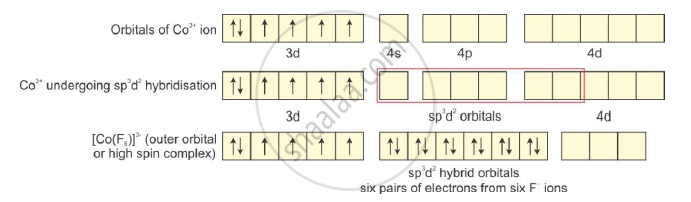
On The Basis Of Valence Bond Theory Explain The Nature Of Bonding In Cof6 3 Ion Chemistry Shaalaa Com
Draw molecular orbital diagram for F 2 molecule. Also, gives its electronic configuration, bond order and magnetic property. Hint: The Molecular Orbital Theory (MOT) explains the formation of the molecule in a better way than Valence Bond Theory (VBT). The bond order calculations are feasible using MOT and so is the description of electronic ...





















0 Response to "42 orbital diagram of f- ion"
Post a Comment