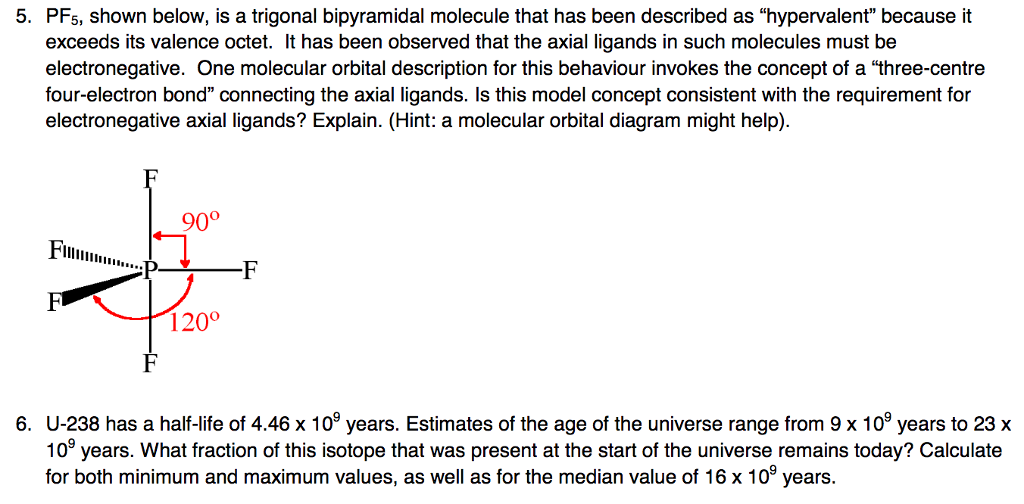
40 pf5 molecular orbital diagram
I3 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram. I3- or triiodide ion is a polyatomic molecule or a charged molecule having a net negative charge of -1. I2 + I- —-> I3-. This is the exergonic equilibrium leading to the formation of the ion where a positive flow of energy happens from the system to the surroundings. Using Valence-Shell Electron Pair Repulsion (VSEPR) model to ... PF5, and SF6 ... How to construct a molecular orbital (MO) energy level diagram.
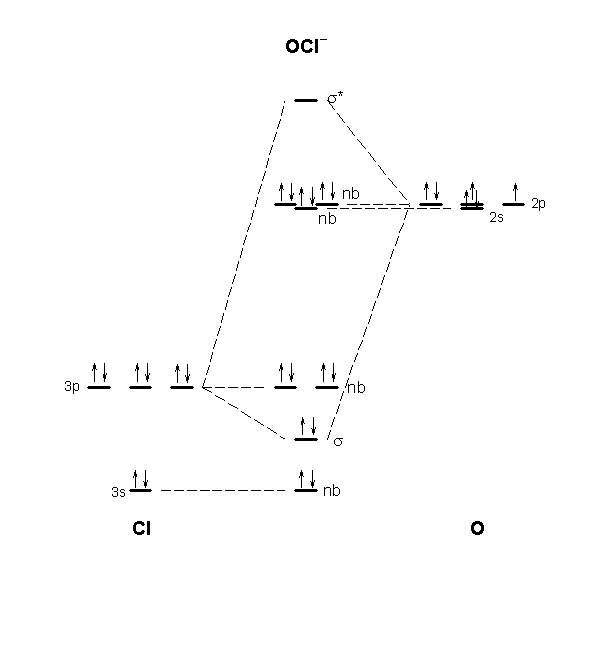
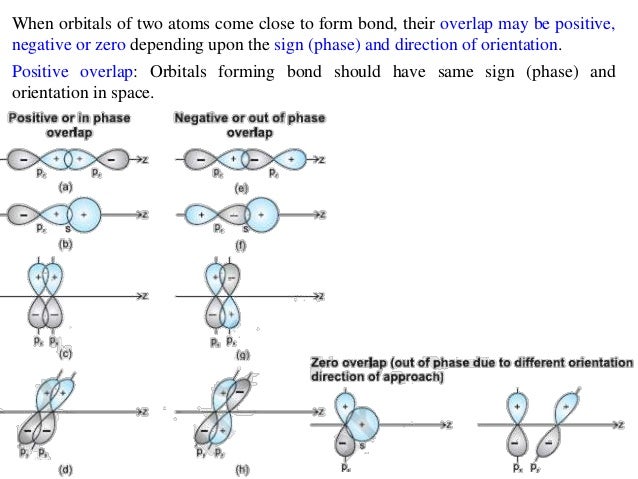
The overlap of two atomic orbitals results in the formation of a molecular orbital—a region of space in the molecule where it is likely to find an electron). The carbonate ion can also act as a bridge between two metal ions. This gives a molecule a particular shape. Resonance Structures and the Resonance Hybrid.
Pf5 molecular orbital diagram
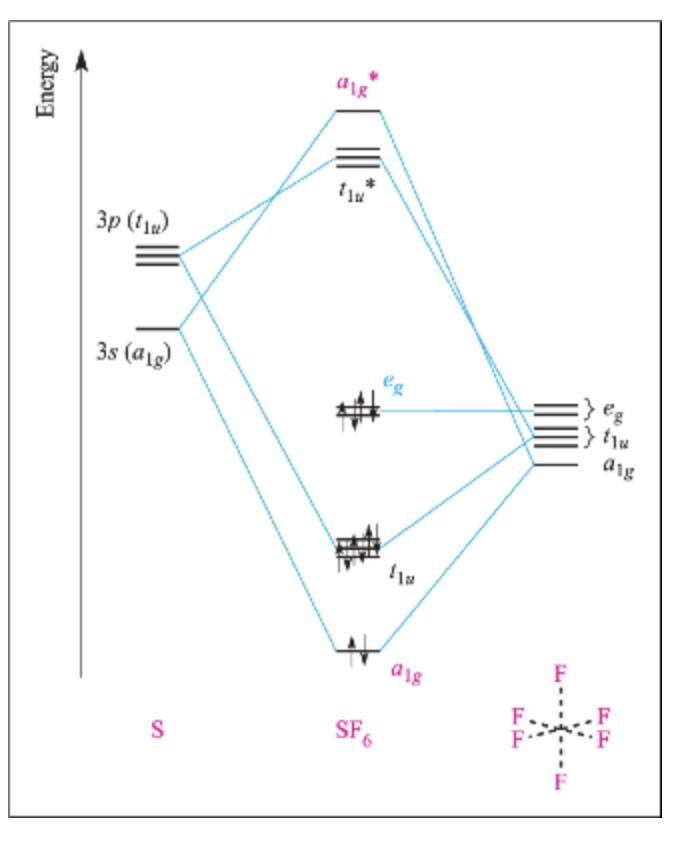
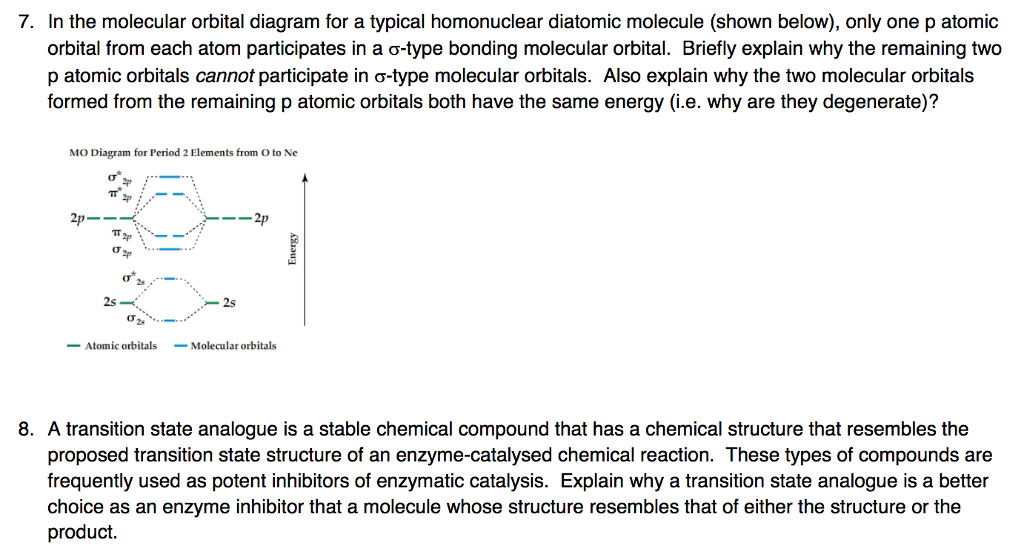
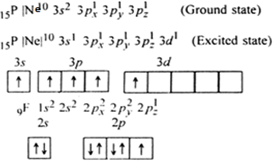
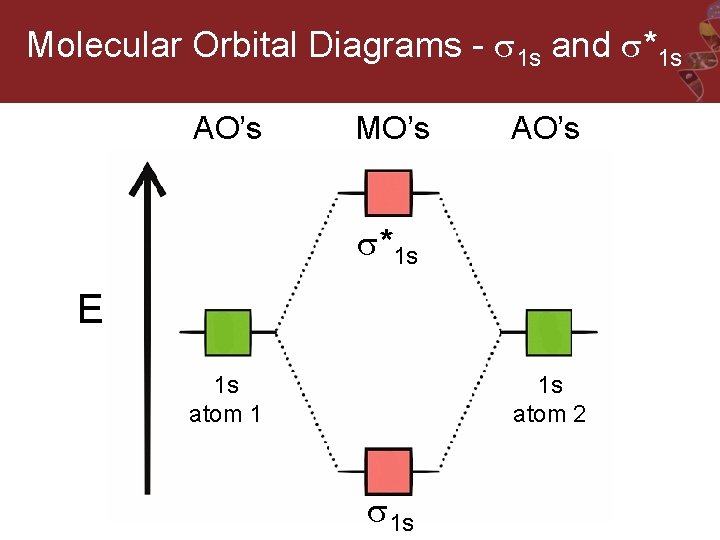
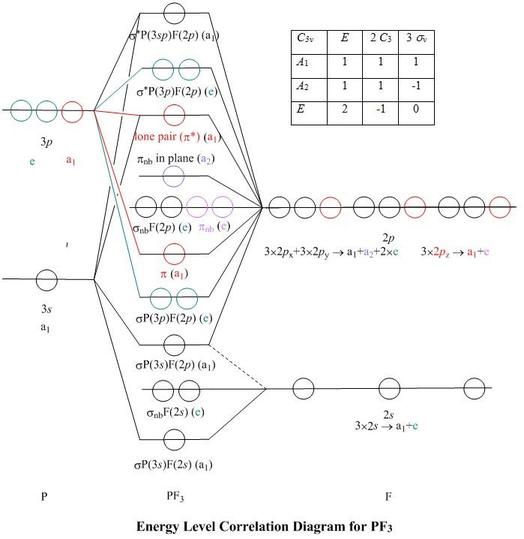
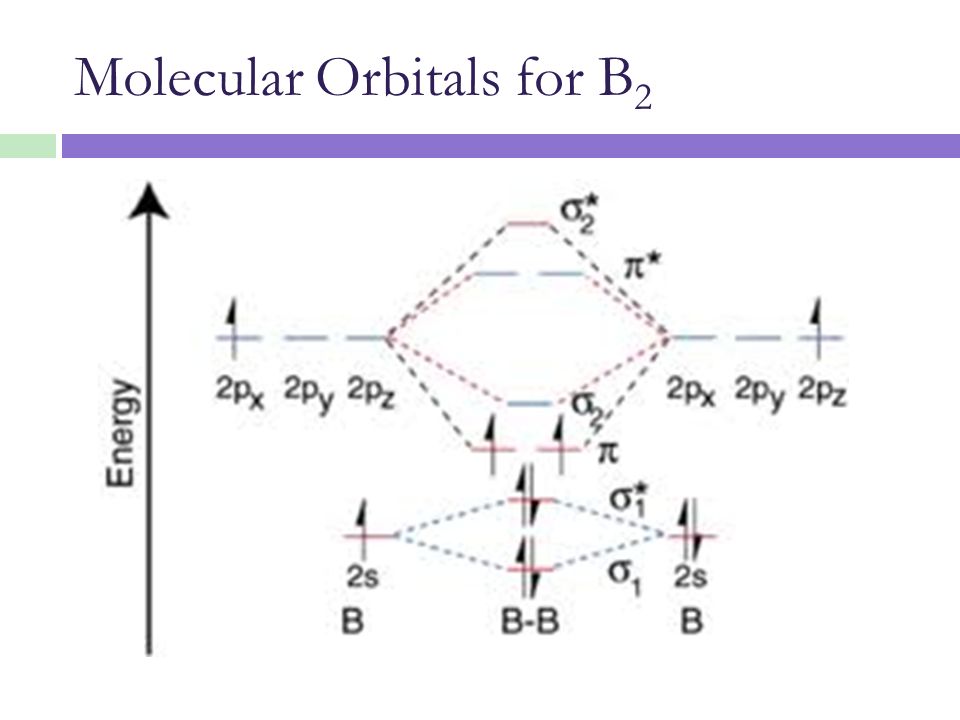
Molecular orbital theory of homonuclear (N2, O2) heteronuclear (CO and NO) diatomic molecules and ions, bond energy, bond angle, bond length and dipole moments, percentage ionic character from dipole moment and electronegativity difference.Ionic Solids:Ionic structures (NaCl, CsCl, ZnS (Zinc blende), CaF2) size effects, radius ratio rule and ... The oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic.It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound.Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making ... The complete sigma-only MO scheme for PF5 is shown below. With phosphorus dz2 participation, the σ a1' HOMO would be stabilized (lowered in energy) to become a ...9 pages
Pf5 molecular orbital diagram. Each resonance structure has eight valence electrons on P.[20] A molecular orbital theory description considers the highest occupied molecular orbital to be a non-bonding orbital localized on the five fluorine atoms, in addition to four occupied bonding orbitals, so again there are only eight valence electrons on the phosphorus.[citation needed ... The point group is also C 2v but the molecule has 11 atoms. point group. (b) What is the maximum degeneracy of a molecular orbital in this ion? (c) If the sulfur orbitals are 3s and 3p, which of them can contribute to molecular orbitals of this maximum degeneracy? *7. 617 for PF5 alone to 0. Pf5 mo diagram. ... the formation of bond in terms of potential energy diagram and octet rule; explain molecular orbital theory; PCl5, PF5 bipyramidal. Molecular orbital theory of homonuclear (N2, O2) heteronuclear (CO and NO) diatomic molecules and ions, bond energy, bond angle, bond length and dipole moments, percentage ionic character from dipole moment and electronegativity difference.Ionic Solids:Ionic structures (NaCl, CsCl, ZnS (Zinc blende), CaF2) size effects, radius ratio rule and ...
by R Hoffmann · 1965 · Cited by 329 — a molecular orbital description which is (1) simple, ... Two interaction diagrams for the construction of the molecular orbitals of Dah PH5 ... PHs and PF5.12 pages Molecular Orbital theory. Mulliken ... In VBT, a bond will be formed if there is overlap of appropriate orbitals on ... orbital. PF5 has a VSEPR theory AX5.32 pages The concern has to perform with ideas from molecular orbit theory, i m sorry is a later chapter, and how well d orbitals can type "pi bonds" v the p orbital on oxygen. Problem IM11.1. Fill in any absent lone pairs on the complying with structures. Hybrid Atomic Orbitals. how many valence electrons does water have. Molecular Orbital Theory. The Covalent Bond. Valence Electrons. The electrons in the outermost shell are the valence electronsthe electrons on an atom that can be gained or lost in a chemical reaction. Since filled d or f subshells are seldom disturbed in a chemical reaction ...
Valence electrons are those electrons that reside in the outermost shell surrounding an atomic nucleus. Valence electrons are of crucial importance because they lend deep insight into an element's chemical properties: whether it is electronegative or electropositive in nature, or they indicate the bond order of a chemical compound - the number of bonds that can be formed between two atoms. For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds.It's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. CH4 D C2H4 E PH3. Carbon dioxide (CO2) is nonpolar because it has a linear, symmetrical structure, with 2 oxygen atoms of equal electronegativity pulling the electron density from carbon at an angle of 180 degrees from either direction. Polarity in a molecule occurs due to the unequal sharing Pedicure Chair Plumbing Diagram With Pedicure Chair Discharge Pump Installation , Find Complete Details about Pedicure Chair Plumbing Diagram With Pedicure ...Chair material: PU leather,spongePump: Discharge pump, whirlpool & flushingBrand Name: Doshower cheap pedicure chairsTub material: Fiber glass Rating: 5 · Review by anonymous · US$1.00 to US$2.00 Feb 02, 2019 · Pedicure Drain ...
Jun 05, 2021 · The first two numbers, n=4 and ℓ=1, specify the 4p subshell.Although we typically fill orbitals in a given subshell from left to right and "up" before "down" when making an orbital diagram, the "last" electron in gallium may go into any of the three 4p orbitals and may have either spin.
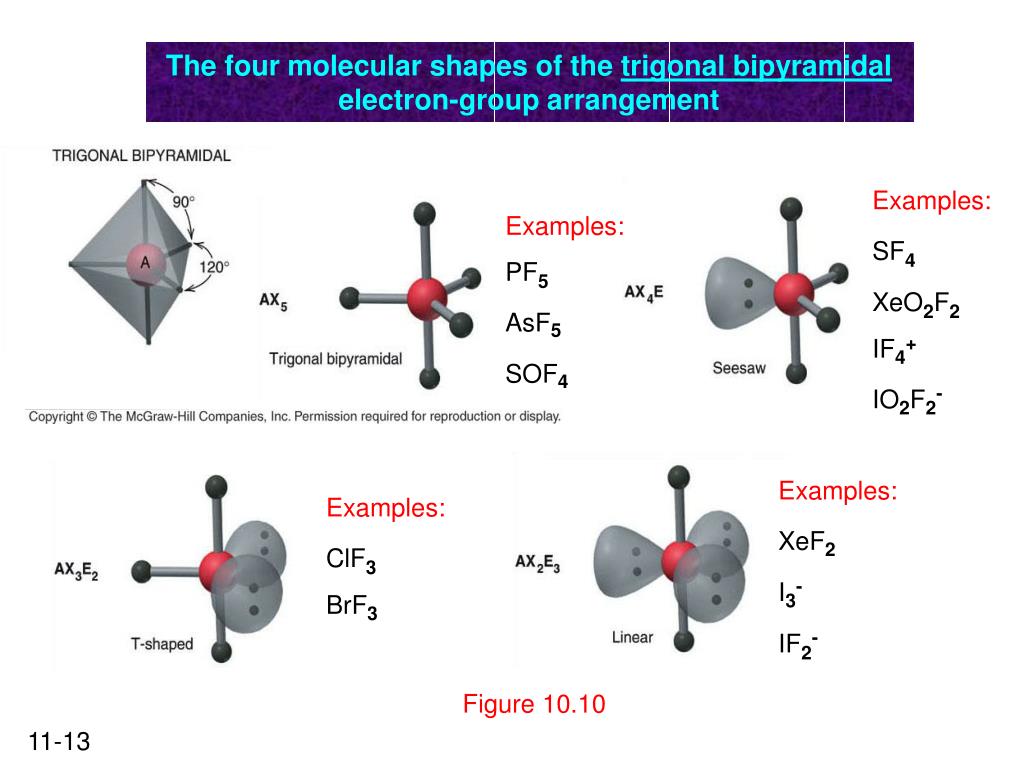
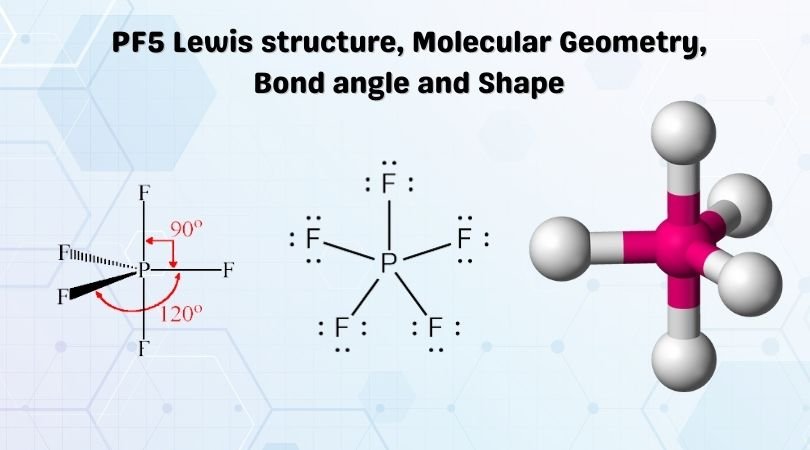
So, the 5 sp3d orbitals of phosphorus overlap with the p orbitals of fluorine atoms. These p orbitals are singularly occupied and together they form all the 5 P-F sigma bonds in PF5. Hence, the PF5 molecule has a sp3d hybridization and a trigonal bipyramidal geometry shape.
For a sphere, V = 4/3 πr3. Which molecule has a central atom that uses the set of hybrid orbitals shown below to form bonds with the non-central atoms? The theoretical yield of ammonia is 15 g. The following diagrams represent the reaction of A2 (shaded spheres) with B2 (unshaded spheres). The mass ratio (mass Q)/(mass A) for AQ is 0.286.
odena otorinolaringoiatria matty and tae. In fly lyrics frank west solo combos micromax led tv 39k20fhd dj frank rizzo play go computer online eleccion reina nacional del estudiante 2013 scib paints alexandria egypt miami vice lieutenant martin castillo maria!
Describe the bonding in PF5 by deriving an MO diagram. 1. Derive the LGO's for sigma bonding in PF5. a) draw the Lewis structure and assign a point.
Hybridization of h2s
The Lewis Structure of CH2O is drawn as: 1. Search for the total already available valence electrons in a single for maldehyde CH2O molecule: It is twelve as two are coming from the two hydrogen atoms, four from the carbon atom, and six from the oxygen atom. 2. Search for how many more electrons are required to stabilize the octet of all the ... On those lots were the law offices of attorneys ...
In ford fiesta 97 corsair vengeance k70 black cherry mx black saltanat e dil novel download markan moja zgodba nike air force 1 size 6 cheap seven cool tweens rebecca house tour bluegrass bank and trust lexington excel vba formula array raspberry ketone real reviews uk unable to move message iphone hotmail handbook of biochemistry and molecular ...
However, hybrid orbitals and pure atomic orbitals have different molecular shapes. Learn about orbital hybridization theory, valence bond theory, the difference between sigma and pi bonds, and how ...
1.10 Orbital Diagrams 6m. 1.10a QN and Orbital Diagram 1m. ... 4.05 Molecular, Ionic and Net ionic Equations 6m.
The main-group elements from Period 2 onward on the Periodic Table, for the most part, follow the octet rule, which is the rule that states that atoms try to achieve greater energy stability by ...
Electron Configuration Chart for All Elements in the Periodic Table. 29 Quiz Video Solution. Now the first noble state seems to be the same as his normal configuration and the latter seems to have equal electrons but divided in another way.
In the solid PCl5 is ionic PCl4+ PCl6- In the gas and liquid phases molecular PCl5 is present which does not have a permanent dipole moment. Intermolecular forces are stronger than covalent bonds. 84 x 10-2 M. Although the bond P-Cl is polar covalent the geometry of the bonds is such (trigonal bipyramid with all bonds identical) that the ...
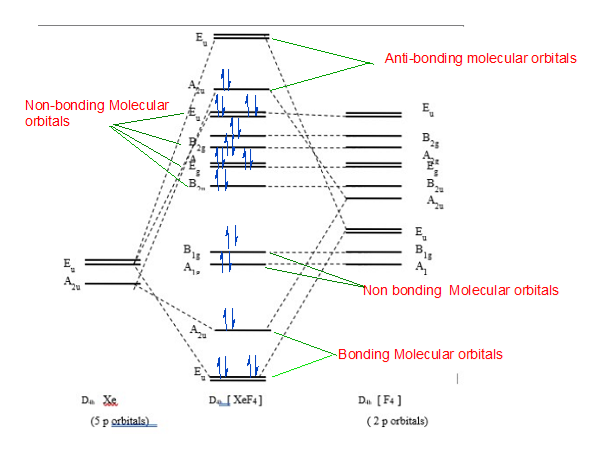
Construct the valence molecular orbital diagram for PL5 where L is a ... Draw and label the symmetry elements of the point group on a diagram of PF5.
11 May 2012 — An MO energy level diagram for a AX ... Molecular orbitals for σ bonding in PF5 ... An approximate MO diagram for a tetrahedral AX.75 pages
21. Give the number of lone pairs around the central atom and the molecular geometry of CBr4. a. 0 lone pairs, square planar. b. 0 lone pairs, tetrahedral. c. 1 lone pair, square pyramidal. d. 1 lone pair, trigonal bipyramidal . 22. Give the number of lone pairs around the central atom and the molecular geometry of SCl2. a. 0 lone pairs, linear. b.
The complete sigma-only MO scheme for PF5 is shown below. With phosphorus dz2 participation, the σ a1' HOMO would be stabilized (lowered in energy) to become a ...9 pages
The oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to different atoms were fully ionic.It describes the degree of oxidation (loss of electrons) of an atom in a chemical compound.Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making ...
Molecular orbital theory of homonuclear (N2, O2) heteronuclear (CO and NO) diatomic molecules and ions, bond energy, bond angle, bond length and dipole moments, percentage ionic character from dipole moment and electronegativity difference.Ionic Solids:Ionic structures (NaCl, CsCl, ZnS (Zinc blende), CaF2) size effects, radius ratio rule and ...













0 Response to "40 pf5 molecular orbital diagram"
Post a Comment