45 bohr diagram of helium
Does Bohr model work for helium? The usefulness of Bohr’s theory extends beyond the hydrogen atom. Bohr himself noted that the formula also applies to the singly ionized helium atom, which, like hydrogen, has a single electron. The nucleus of the helium atom has twice the charge of the hydrogen nucleus, however. Sep 01, 2021 · Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, well show a sample Bohr diagram for hydrogen. H Hydrogen. 0 neutrons. You can see the principles outlined in the section above at work in the Bohr model for the hydrogen atom.
Video explains how to use the Periodic Table components to create a Helium Atom Bohr Model. Learn how to determine the number of protons from the Atomic Num...
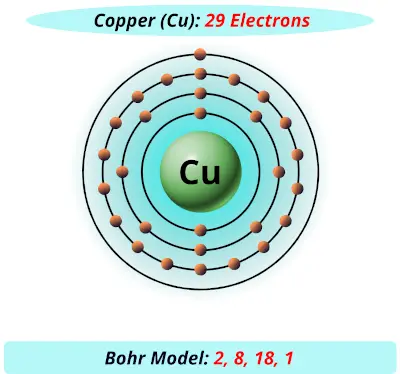
Bohr diagram of helium
Bohr Model Practice 1. Carbon , 4. Chlorine 7. Aluminum 10. N'ì NAME BLOCK 3. Oxygen 6. Neon 9. Helium 12. Beryllium 2. Hydrogen 009 5. Sodium A singly ionized helium atom is hydrogenic in that it consists of a nucleus (two protons and two neutrons) orbited by a single electron and thus the Bohr theory should be … Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8 ...
Bohr diagram of helium. In Old Bohr's circular helium, electrons are kicked out from orbits due to destructive interference between opposite de Broglie wave phases. Actually, this old Helium model with a single circular orbit containing two electrons gives wrong ground state ene rgy (= -83.33 eV ) which is lower than true Helium energy (= -79.004 eV ). The Bohr model of Helium is drawn with only one electron shell and it contains 2 electrons. Helium is neutral and its atomic number is 2, hence, the number of protons and electrons available for its Bohr diagram is also 2. The number of neutrons for the Bohr diagram of Helium can be found by subtracting the number of protons from the atomic ... Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He Sep 24, 2019 · Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we’ll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
The Bohr model gives almost exact results only for a system where two charged points orbit each other at speeds much less than that of light. This not only involves one-electron systems such as the hydrogen atom, singly ionized helium, and doubly ionized lithium, but it includes positronium and Rydberg states of any atom where one electron is far away from everything else. Helium Oxygen Fluorine Nitrogen Silicon . Lewis Dot Diagram Worksheet Use the Bohr models to determine the number of valance electrons. Once you have found the number of valance electrons, place them around the elements symbol. Element Atomic # Atomic Mass Protons Neutrons Electrons ... Jul 28, 2016 · 3- Bohr’s theory successfully explained the observed spectra for H – atom and similar ions (He+1 , Li+2 , Be+3 etc) but it can not explained the spectra for poly electron atoms. Prepared by: Sidra Javed Hydrogen 1p, 1e Helium ion 2p, 1e +1 Beryllium Ion 4p, 1e +3 Lithium Ion 3p, 1e +2 40. Aug 15, 2020 · Bohr diagrams. Bohr diagrams indicate how many electrons fill each principal shell. Group 18 elements (helium, neon, and argon are shown) have a full outer, or valence, shell. A full valence shell is the most stable electron configuration.
where RH is the Rydberg constant, Z is the atomic number, and λ is the wavelength of light emitted, could be explained by the energy differences between the quantized electron energies n.Since the Bohr model applies to hydrogen-like atoms, i.e., single-electron atoms, for the case of He+, Z=2 and RHZ2 = 4.38949264 x 107 m-1.We can use this equation to calculate the ionization potential of He+ ... Oct 24, 2018 · diagram for CO2 in Figure can be used as a guide, with the orbitals of Be higher in energy than those of C and the orbitals of F lower in energy than those of O. Calculated molecular orbital shapes are below, for comparison for those of CO 2 in Figure The energy-level diagram for He2 is shown above, the two electrons in each of the 1s atomic ... The helium atom has 2 electronic energy levels: E3p = 23.1 eV and E2s = 20.6 eV where the ground state is E = 0. If an electron makes a transition from 3p to 2s, what is the wavelength of the photon emitted? E γ = E3p −E2s = 2.5 eV (1) So λ = hc E γ = 1240 eV −nm 2.5 eV ≈ 500 nm (2) This is blue-green light. Bohr Model of the Hydrogen Atom Helium Atom Diagram — UNTPIKAPPS (Isabel Roberts) Bohr's model agreed with the hydrogen spectrum results, but did not agree with the spectrum of Helium. In Old Bohr's circular helium, electrons are kicked out from orbits due to destructive interference. Helium is a remarkable and unique element.
Figure 1. Niels Bohr, Danish physicist, used the planetary model of the atom to explain the atomic spectrum and size of the hydrogen atom. His many contributions to the development of atomic physics and quantum mechanics, his personal influence on many students and colleagues, and his personal integrity, especially in the face of Nazi oppression, earned him a prominent place …
Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr model of Neon (Ne) 2, 8: 11: Bohr model of Sodium (Na) 2, 8 ...
A singly ionized helium atom is hydrogenic in that it consists of a nucleus (two protons and two neutrons) orbited by a single electron and thus the Bohr theory should be …
Bohr Model Practice 1. Carbon , 4. Chlorine 7. Aluminum 10. N'ì NAME BLOCK 3. Oxygen 6. Neon 9. Helium 12. Beryllium 2. Hydrogen 009 5. Sodium







0 Response to "45 bohr diagram of helium"
Post a Comment