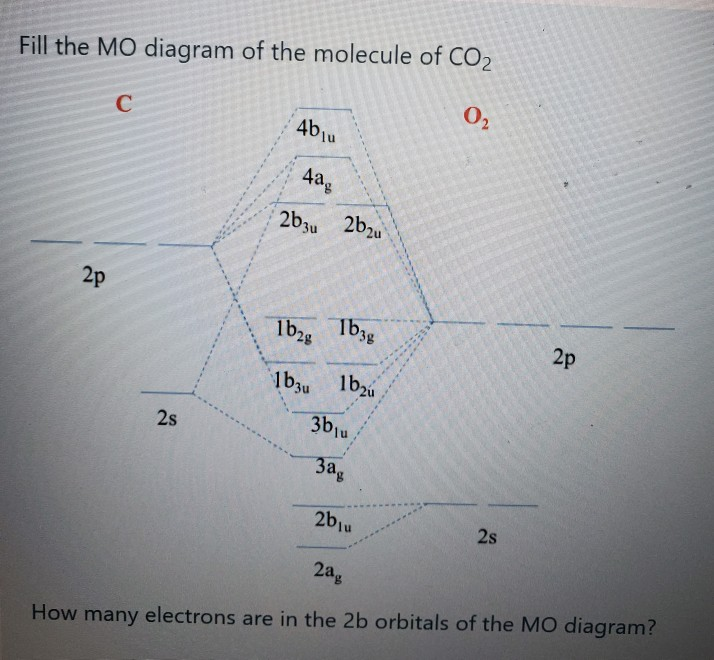
43 mo diagram for co2
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Sep 7, 2020 — First of all MOT theory is applicable only for diatomic molecules so MO diagram for CO2 is not possible to draw. Now coming to main question it's not ...3 answers · 1 vote: The atomic orbitals of oxygen are uniformly lower in energy than the corresponding atomic ...How is the bond order of CO2 determined? - Quora12 answersJun 1, 2016What is the molecular orbital energy diagram of CO ...4 answersJan 7, 2017What is the bond order of CO? - Quora14 answersSep 16, 2016Why is the molecular orbital diagram for O₂ ...2 answersAug 1, 2019More results from www.quora.com
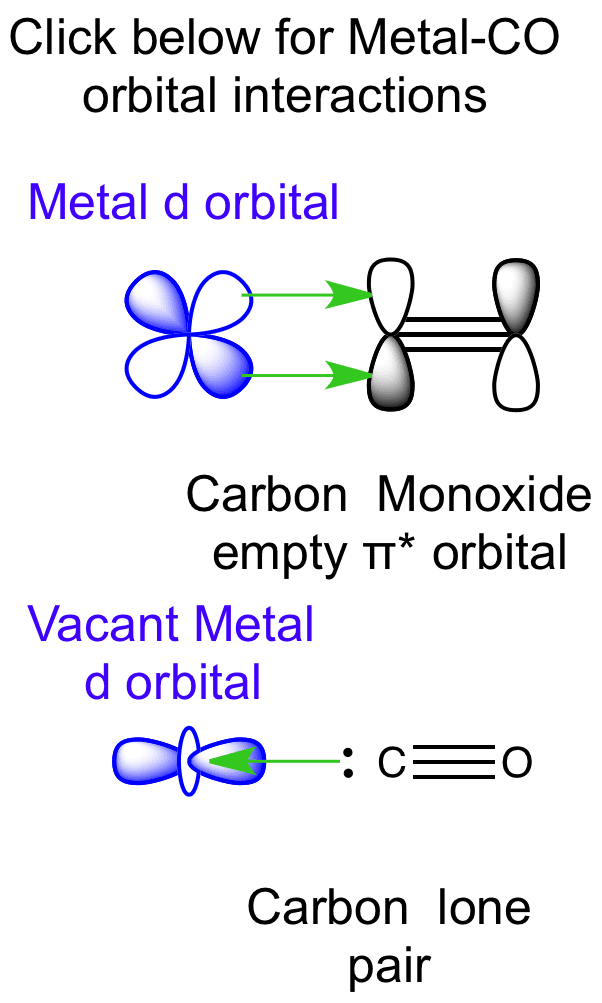
Molecular Orbital (MO) Diagram In a Molecular Orbital Diagram, the 2s orbital of oxygen is nonbonding because of the high energy difference between carbon and oxygen atoms. Based on the rules of the Lewis Structure, all 16 electrons are filled upon bond formation, but the nonbonding orbitals remain vacant, as in the case of CO2.
Mo diagram for co2
CO2 MOs MO Diagram for CO2 C 2p C 2s bonding MOs antibonding MOs C AOs O LCAOs 1 3 u 3 g 2 u 1 g 1 u 2 u 2 g 1 u 1 g 15. Molecular orbital theory for SF6 molecule- • Electronic configuration of sulphur: • Electronic configuration of Fluorine: • Total number of valence electron: • Hybridization: • Structure of SF6- Feb 3, 2021 — The carbon dioxide MO diagram is based on a C atom and an O--O ligand fragment. Carbon has 2S and 2Px,y,z orbitals and the O--O fragment has 2S ...Introduction · MO Theory · Carbon Dioxide MO diagram Carbon dioxide (CO2) molecular orbital diagram (Jmol visualization) for important MOs. The HOMO-1, HOMO and LUMO+1 are degenerate and the HOMO-5, HOMO-4 and HOMO are non-bonding lone pairs. Firefly 8.2.0 DFT B3LYP 6-311++G(d,p) geometry-optimized structure (energy minimum, no imaginary frequencies). The degenerate orbitals are perpendicular to each other. Total energy = -188.6469 Hartrees. C=O ...
Mo diagram for co2. Below is the MO diagram for CO2. a. Complete the MO diagram by adding the electrons. b. When two electrons are added to the CO2 does the bond order increase or decrease; explain. c. What effect does removing an electron have on the bond order of CO2; explain. mo diagram of pie bonded moleculeFollow me on instagram-https://www.instagram.com/trickychemistrysuman/?hl=enFollow me on facebook page-https://lm.facebook.c... Carbon Dioxide by Reducible Representations Γ2s= Ag+ B1u Γ2pz= Ag+ B1u Γ2px= B2g+ B3u Γ2py= B3g+ B2u B3u B2u Ag Ag 2p x 2p y 2p z 2s B2g B3g B1u B1u These are the same group orbital symmetries that we got using inspection. We can (re)draw them. 5. Find matching orbitals on central atom Ag B1u B3u B2u 6. Build MO diagram… Molecular Orbital Diagram for Oxygen Gas (O2).Fill from the bottom up, with 12 electrons total.Bonding Order is 2, and it is Paramagnetic.sigma2s(2),sigma2s*...
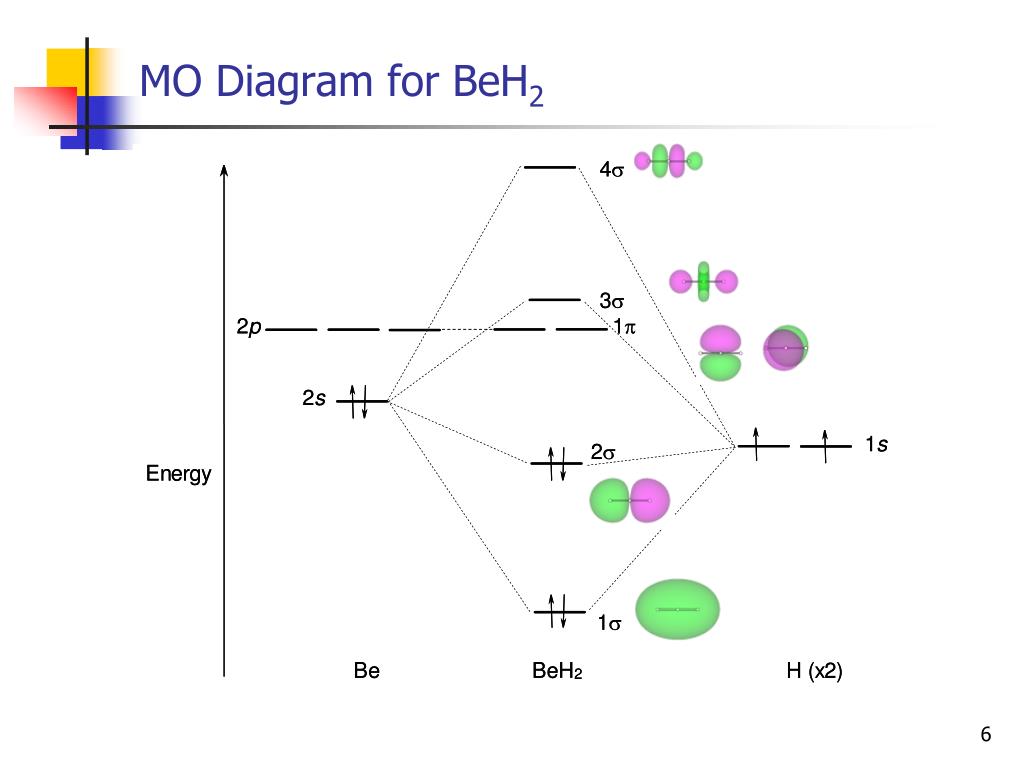
Question 1 To build the MO diagram for CO2, we will consider the s and p orbitals on C and the two O's. How many oxygen SALCs are there? Question 2 12 pts Part A. Draw the oxygen SALCs. (2 pts) Part B. Placing the atomic orbitals of carbon on the left side and the SALCs of oxygen on the right side, draw a MO diagram for CO2. Although this is a 6.2.2: Carbon dioxide. Construct SALCs and the molecular orbital diagram for CO 2. Step 1. Find the point group of the molecule and assign Cartesian coordinates so that z is the principle axis. Step 2. Identify and count the pendant atoms' valence orbitals. Step 3. Generate the Γ 's. Step 4. MO Diagram CO2. Draw similar diagrams for other orbitals in the print out. Make sure you understand how the molecular orbital coefficients are determined and their meaning. Draw similar diagrams for other orbitals in the print out.The first photo is straight from a edition Pearson general chemistry textbook, and it shows you what the molecular ... Carbon dioxide (CO2), molecule is triatomic and linear like Beryllium di hydride (BeH2) However, unlike hydrogen as peripheral atoms in BeH2, there are oxyge...
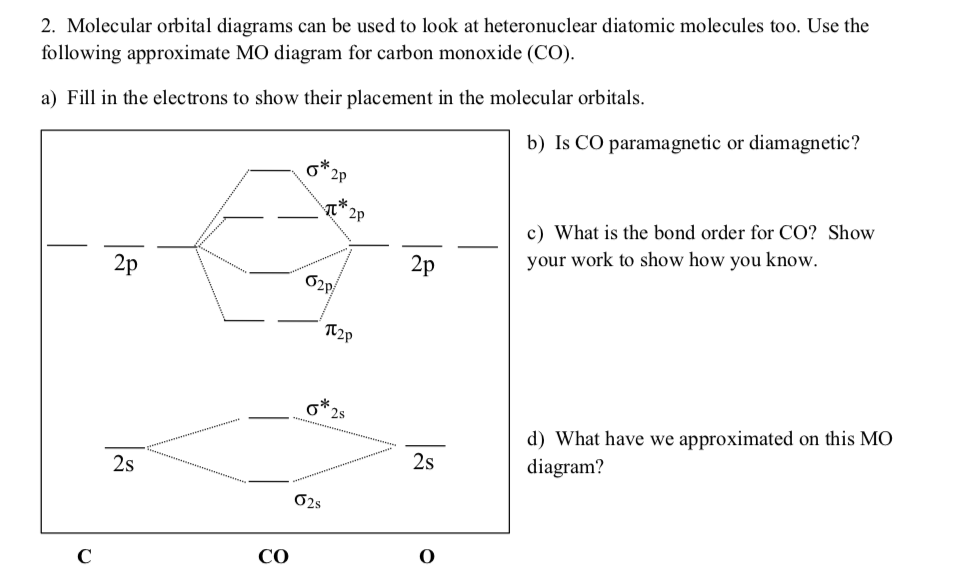
Carbon dioxide is electron-poor at the central carbon and acts as an electrophile. The molecular orbital diagram of carbon monoxide is very similar to that of ... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... The Molecule. CO is a very stable 10-valence-electron molecule, isoelectronic with [CN] - and with N 2, which has a slightly lower bond dissociation energy than CO. The formal bond order of CO is 3, from about one σ- bond and two π- bonds. Its most important property is burning in air to give CO 2 , in the combustion of fossil fuels. Answer (1 of 3): The atomic orbitals of oxygen are uniformly lower in energy than the corresponding atomic orbitals of element C because of the increased stability of the electrons in oxygen. The molecular orbitals are no longer symmetrical, and the energies of the bonding molecular orbitals are ...
File:MO Diagram CO2.svg. Size of this PNG preview of this SVG file: 406 × 599 pixels. Other resolutions: 162 × 240 pixels | 325 × 480 pixels | 406 × 600 pixels | 520 × 768 pixels | 694 × 1,024 pixels | 1,387 × 2,048 pixels | 420 × 620 pixels.
Watch the video solution for the question: Draw the orbital diagram for ion Co 2+.. . can be accommodated in the metal d orbitals. • d0 ions •d7 ions - Fe1+, Ru1+, Co2+, Rh2+, Ni3+, etc.. σ-ML4 Tetrahedral MO Diagram e. Answer to Write orbital diagram for Co2+. Use the buttons at the top of the tool to add orbitals.
Apr 25, 2020 · 1 answerQuestion #112252. Draw molecular orbital diagram for CO2. 1. Expert's answer. 2020-04-27T01:53:22-0400. Molecular Orbital Diagram for CO2.
Jmol models of wavefunctions calculated at the RHF/3-21G* level. To view a model, click on an orbital in the energy level correlation diagram shown ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining MO diagrams can explain why some molecules exist and others do not. . combinations such as CO and NO show that the 3σg MO is higher in energy. Mulliken came up with theory known as Molecular Orbital Theory to explain questions like above.
CO2 doesn't have any lone pair, so both geometries are the same in this case. Let's move on to the molecular orbital diagram of this compound. CO2 Molecular Orbital (MO) Diagram. The molecular orbital diagram of CO2 is as below. A molecular orbital diagram of any compound gives us an idea about the bonding of the orbitals.
Carbon dioxide (CO2) molecular orbital diagram (Jmol visualization) for important MOs. The HOMO-1, HOMO and LUMO+1 are degenerate and the HOMO-5, HOMO-4 and HOMO are non-bonding lone pairs. Firefly 8.2.0 DFT B3LYP 6-311++G(d,p) geometry-optimized structure (energy minimum, no imaginary frequencies). The degenerate orbitals are perpendicular to each other. Total energy = -188.6469 Hartrees. C=O ...
Feb 3, 2021 — The carbon dioxide MO diagram is based on a C atom and an O--O ligand fragment. Carbon has 2S and 2Px,y,z orbitals and the O--O fragment has 2S ...Introduction · MO Theory · Carbon Dioxide MO diagram
CO2 MOs MO Diagram for CO2 C 2p C 2s bonding MOs antibonding MOs C AOs O LCAOs 1 3 u 3 g 2 u 1 g 1 u 2 u 2 g 1 u 1 g 15. Molecular orbital theory for SF6 molecule- • Electronic configuration of sulphur: • Electronic configuration of Fluorine: • Total number of valence electron: • Hybridization: • Structure of SF6-



.png)

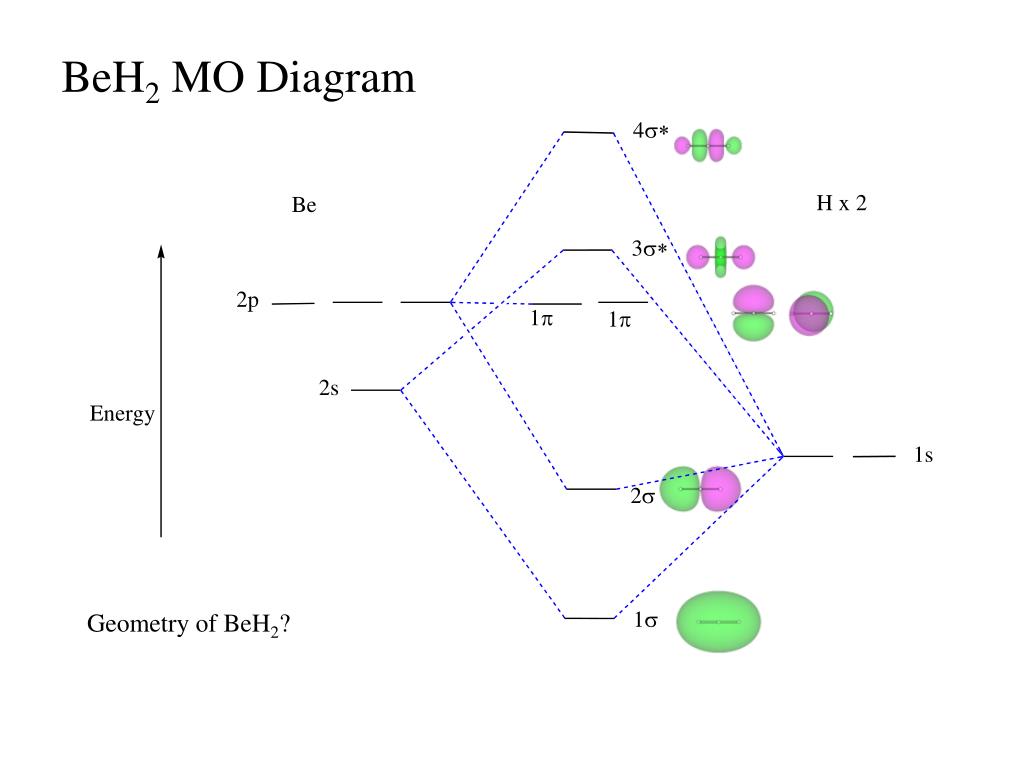


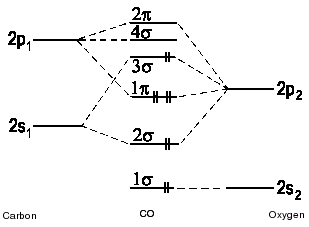






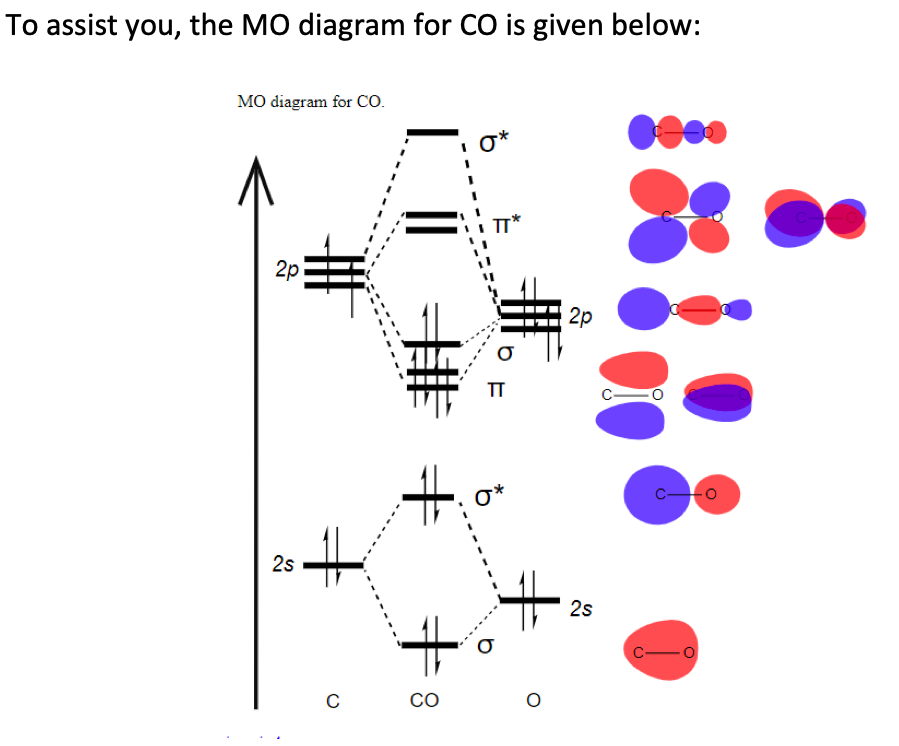










0 Response to "43 mo diagram for co2"
Post a Comment