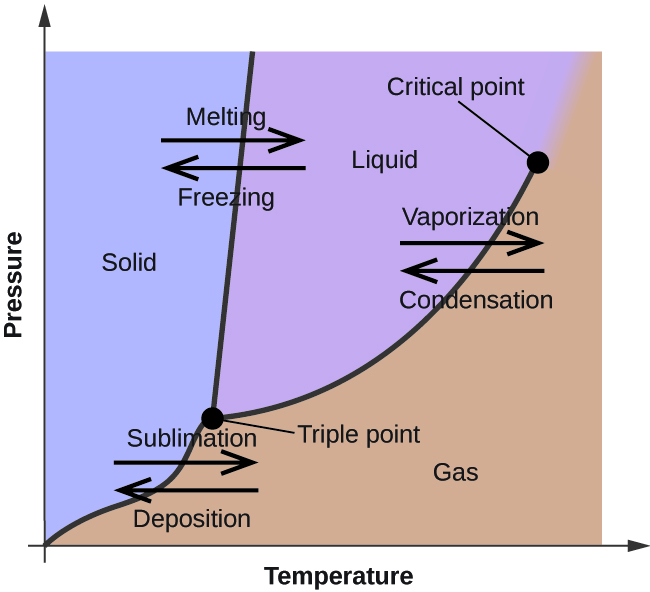
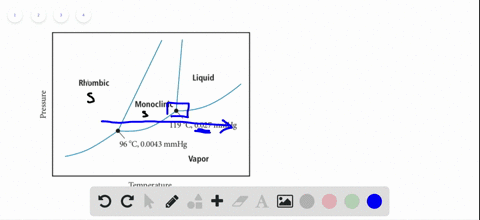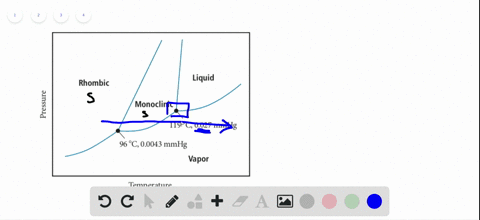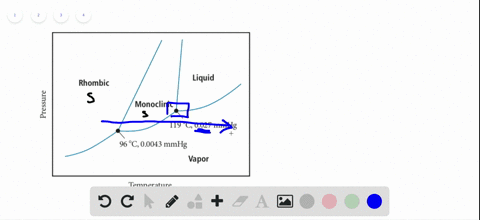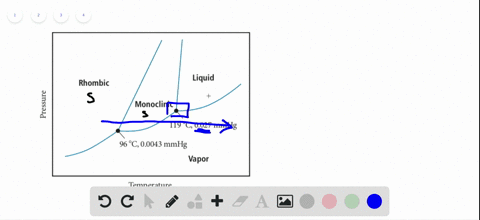
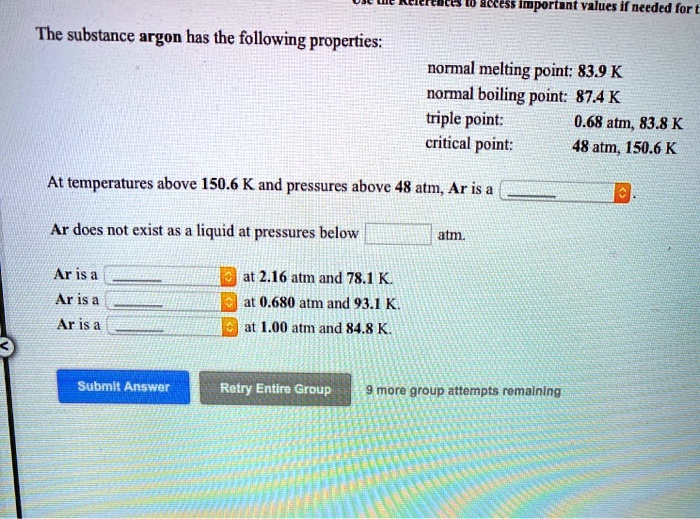
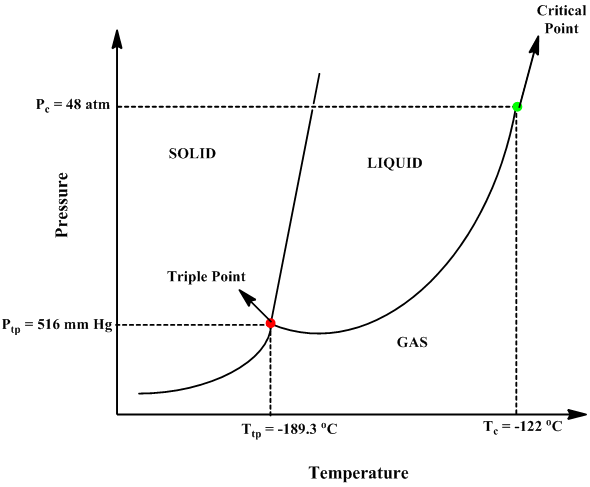
45 sketch the phase diagram to answer whether solid argon or liquid argon has the greater density?
William L. Masterton, Cecile N. Hurley, Edward Neth · 2011 · ScienceThe density of the solid is 1.65 g/cm3, whereas that of the liquid is 1.40 g/cm3. Sketch the phase diagram for argon and use it to fill in the blanks below ... Solid argon hydride (Ar(H2)2) has the same crystal structure as the MgZn2 Laves phase. Liquid argon is used as the target for neutrino experiments and direct dark matter searches. Argon has also been used experimentally to replace nitrogen in the breathing or decompression mix known as...
The density of solid Ar (Ar=40 g/mole) is 1.65 g/ml at 40 K. if the argon atom is assumed to be a sphere of radius `1.54xx10^(-8)cm`, then % of solid Ar is apparently empty space?
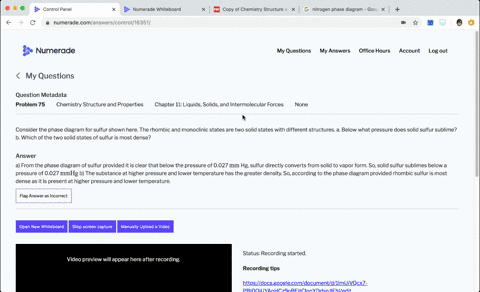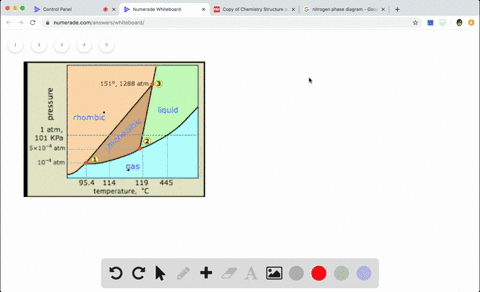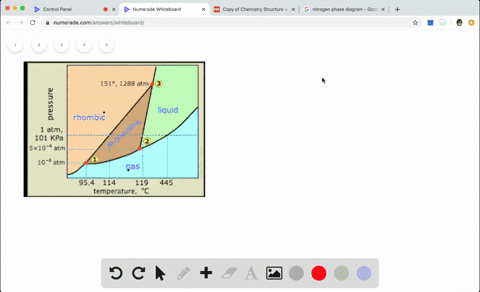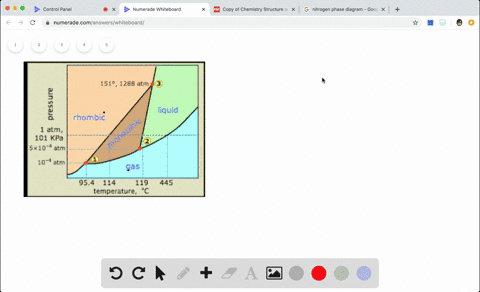
Sketch the phase diagram to answer whether solid argon or liquid argon has the greater density?
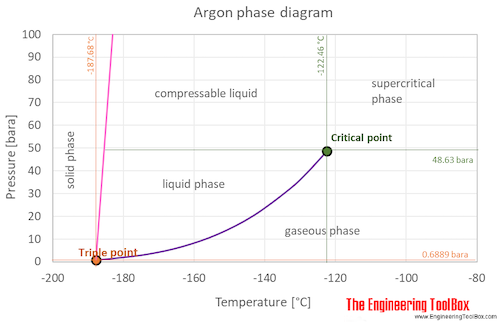
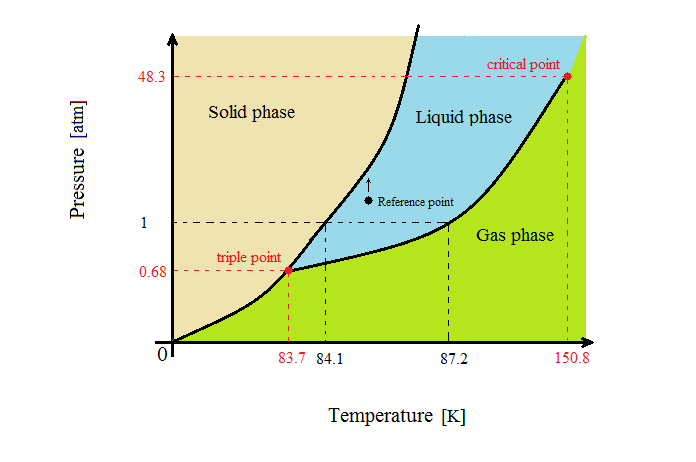
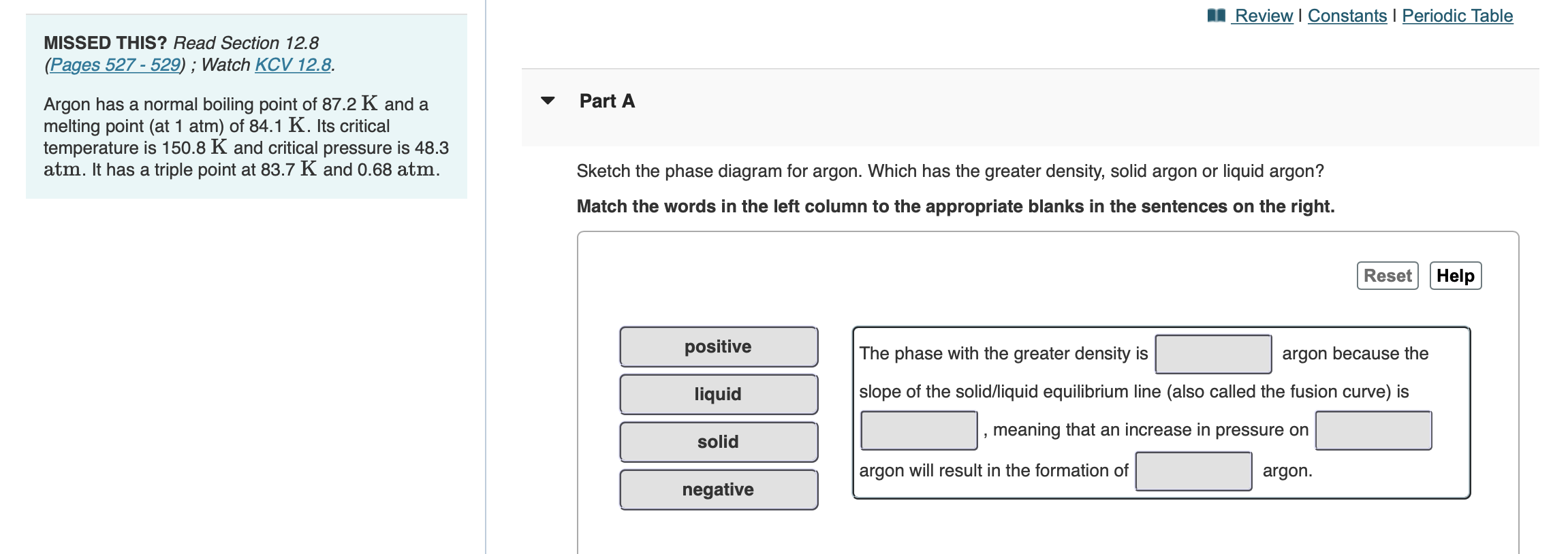
Which has the greater density, solid argon or liquid argon? Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help positive The phase with the greater density is argon because the liquid slope of the solid/liquid equilibrium line (also called the fusion... Toward liquid argon as detection medium. Properties. Neutron and interaction in liquid argon. Mankind has always been fascinated by the origin of the universe. Indeed, despite the great scientic innovation and a wide variety of experimental observations which have changed our understanding of... It has a triple point at 83.7 K and 0.68 atm.Sketch the phase diagram to answer whether solid argon or liquid argon has the greater density?Apr 30, 20191 answer · Top answer: In a phase diagram, the different phases can be identified by their location:• Solid: can be found at high pressure and low temperature• Liquid: ...
Sketch the phase diagram to answer whether solid argon or liquid argon has the greater density?. . If the argon atom is assumed to be a sphere of radius 1.54×10−8. cm, the percentage of empty space in solid argon is: (A )32% (B) 54% (C) 68% (D) 62%. Hint: The volume of one atom of Argon can be calculated by using the following formula. -Means 1.65 g of solid argon has 1 ml volume. Solid or liquid argon have greater density? Matter has more density when solid than when in a liquid state. Argon exists as mono atomic species. The force of attraction will be van der Waals forces of attraction between the argon atoms. The column density of argon is calculated assuming an argon density of 1.77 g cm −3 at 5 K (Dobbs and Jones 1957). ... The phase diagram for argon around the triple point can be found in Figure 2 [77] and [78]. ... The argon phase diagram shows the phase behavior with changes in temperature and pressure. Material Properties - Material properties for gases, fluids and solids - densities, specific heats Argon - Density and Specific Weight - Online calculator, figures and tables showing density and...
The phase relation is often depicted graphically in a phasor diagram. It is sometimes helpful to treat the phase as if it defined a vector in a plane. The usual reference for zero phase is taken to be the positive x-axis and is associated with the resistor since the voltage and current associated with the... CHEM1211 Final Exam Spring 2011 With Answers(1). Chemistry. CHEM1211 Final Exam Spring 2011 With Answers(1). Northeastern University. Use equations or diagrams if they are relevant. ... either freeze or boil. ... (d) Does the liquid phase of argon have a density greater than, equal to, ...4 pages Density of Argon [Ar]. Argon weighs 1.7838 kg/m³ (0.0010311 oz/in³). The search results include links to various calculator pages associated with each found item. Melting Point (MP), Argon changes its state from solid to liquid at -189.37°C (-308.866°F or 83.78K).
Sketch the phase diagram for argon. Which has the greater density, solid argon or liquid argon? Everything in between our liquid and everything below our gas, right is that they say which has the greatest density solid are gun or liquid are gone, Right? Phase Diagram for Water. Water is a unique substance in many ways. One of these special properties is the fact that solid water (ice) is less dense than liquid water just above the freezing point. Notice one key difference between the general phase diagram and the phase diagram for water. Why argon has (or might have) an anomalous dip in radius compared to the trendline It perhaps teaches you how to answer multiple-guess tests the way that undergraduate professors write them The size of argon is greater than chlorine because of the interelectronic repulsions start taking place... It has a triple point at 83.7 K and 0.68 atm. Sketch the phase diagram for argon. Which has the greater density, solid argon or liquid argon?4 answers · Top answer: So here we are asked to draw a face diagram. And typically for face diagrams we plot pressure ...
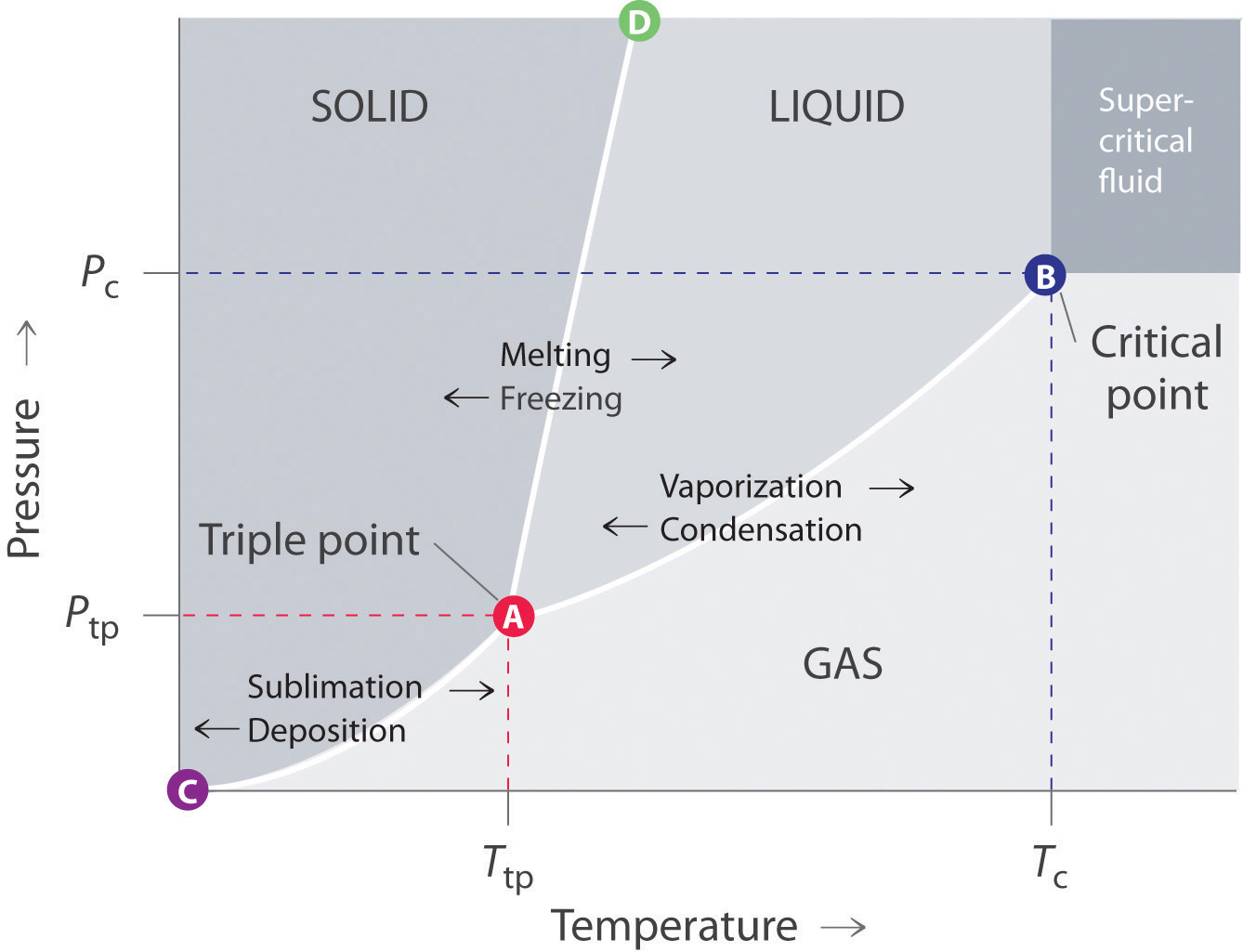
A typical phase diagram has pressure on the y-axis and temperature on the x-axis. This indicates that the liquid phase is more dense than the solid phase. 1. Roughly sketch the phase diagram, using units of atmosphere and Kelvin. Answer.
Assuming that argon has a radius of 190. pm, calculate the density of solid argon. Answer. Aluminum has an atomic radius of 143 pm and forms a solid with a cubic closest packed structure. Sketch the phase diagram for argon and use it to fill in the blanks below with the words "boils"...
Sketch the phase diagram for argon. Which has the greater density, solid argon or liquid argon? From the given information the phase diagram of the argon is as follows -.
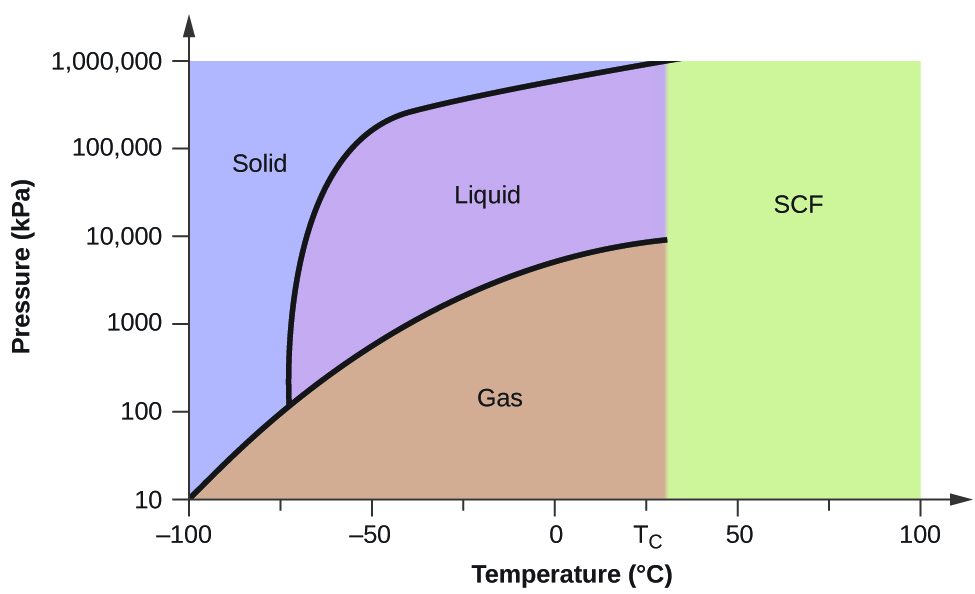
A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid We can use the phase diagram to identify the physical state of a sample of water under specified Like a gas, a supercritical fluid will expand and fill a container, but its density is much greater than typical...
Argon2d uses data-depending memory access, which makes it suitable for cryptocurrencies and PoW applications with no threats from side-channel timing attacks. Scrypt can be a second choice on systems where Argon2 is not available, but keep in mind that it has the same issues with respect to...
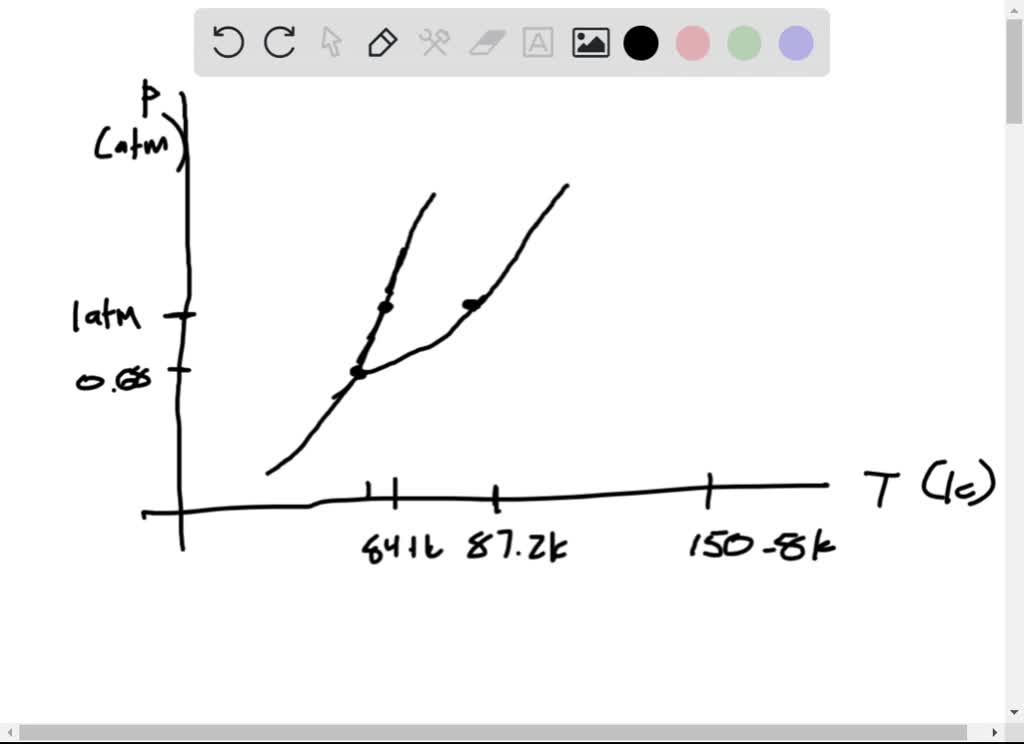
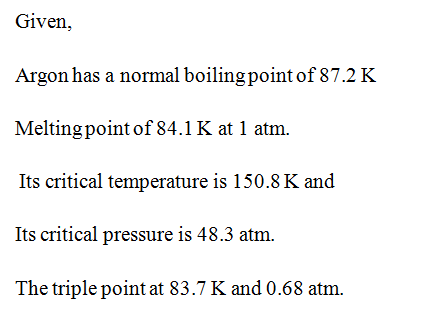
argon has a normal boiling point of 872 k and a melting point at 1 atm of 841 k its critical temperature is 1508 k and its critical pressure is 483 atm it has a triple point at 837 k and 068 atm ske 2
Abstract The density of solid argon has been measured by a bulk density method at temperatures near the triple point (83·8°K). The results have been @article{Smith1967BulkDM, title={Bulk density measurements on solid argon}, author={B. L. Smith and John A. Chapman}, journal={Philosophical...
As a prelude to liquid argon purity measurements, the effect of introducing the contami-nants air, oxygen, carbon dioxide, nitrogen, and water vapour into a highly puried 1 This is particularly signicant when a liquid or a gas comes into contact with a porous solid such as charcoal or aluminium oxide.
No one yet understands why argon has this effect. Brain cells communicate with the use of chemicals called neurotransmitters and with neuroreceptors that fit together like lock and key. Most likely, Nowrangi told Live Science, the gas acts on these neuroreceptors, specifically the NMDA receptor...
Argon, chemical element, inert gas of Group 18 (noble gases) of the periodic table, terrestrially the most abundant and industrially the most frequently used of the noble gases. Thank you for your feedback. Our editors will review what you've submitted and determine whether to revise the article.
Volume of solid argon =1cm3. ∴% Empty space=11−0.380 ×100=62%. The density of gold is 419.3gpercm3.Calculate the diameter of solid gold sphere having a mass of 4239. If all the tetrahedral voids are occupied by B atoms, what is the density (in g cm−3) of resulting solid?[Atomic...
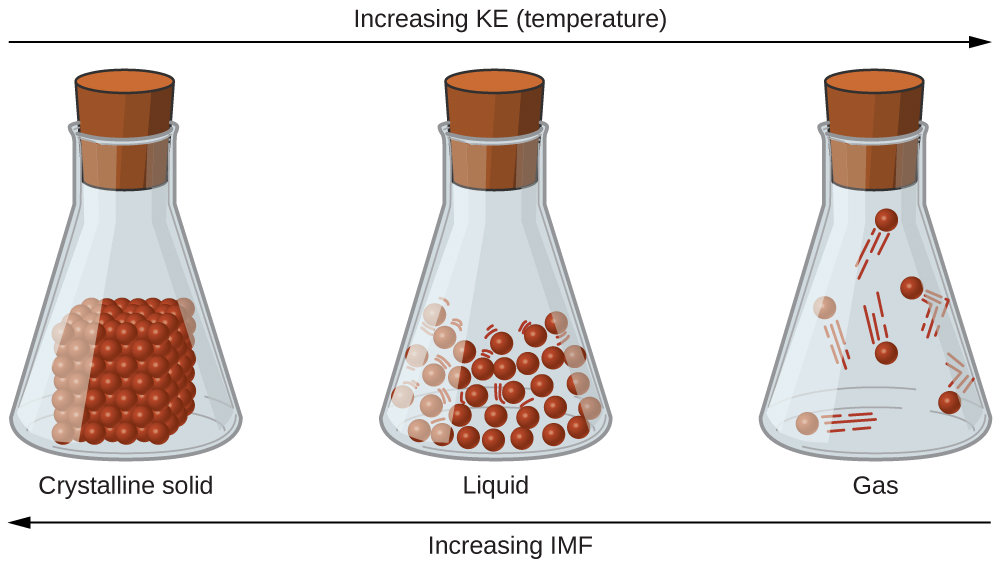
Solids have much greater densities, are compressible only to a very slight extent, and are rigid—a solid maintains its shape irre-spective of its container. *Although the densities of solid and liquid water are quite similar, as is typical for most substances, water is quite unusual in that the density of...
The difference between the densities of solids, liquids and gases is due to the distance between the particles in each state of matter. The particles remain close together, so there is usually only a small increase of volume. The same mass of liquid will have slightly greater volume than the solid.
The solid phase is more dense than the liquid phase. The line that separates solid and liquids bends right. The phase which has the higher density is the phase which exists under higher pressure and lower temperature. Please be sure to answer the question. Provide details and share your research!
The liquid phase is less dense than the solid phase. Answer to nitrogen has a normal boiling point of 773 k and a melting point at 1atm of 631 k. Sketch Does nitrogen have a stable liquid state at 1 atm. Correct exercise 1190 the high pressure phase diagram of ice is shown at the top of the next column.
Sketch the phase diagram to answer whether solid argon or liquid argon has the greater density? solid argon. A mixture containing 22.4 g of ice (at exactly 0.00 ∘C) and 74.2 g of water (at 58.8 ∘C) is placed in an insulated container.
Argon has a normal boiling point of 872 k and a melting point at 1 atm of 841 k. Now go straight up increase in p. Sketch the phase diagram to answer What phase liquid solid or gas is argon. A mixture containing 224 g of ice at exactly 000 c and 742 g of water at 588 c is placed in an insulated...
It has a triple point at 83.7 K and 0.68 atm.Sketch the phase diagram to answer whether solid argon or liquid argon has the greater density?Apr 30, 20191 answer · Top answer: In a phase diagram, the different phases can be identified by their location:• Solid: can be found at high pressure and low temperature• Liquid: ...
Toward liquid argon as detection medium. Properties. Neutron and interaction in liquid argon. Mankind has always been fascinated by the origin of the universe. Indeed, despite the great scientic innovation and a wide variety of experimental observations which have changed our understanding of...
Which has the greater density, solid argon or liquid argon? Match the words in the left column to the appropriate blanks in the sentences on the right. Reset Help positive The phase with the greater density is argon because the liquid slope of the solid/liquid equilibrium line (also called the fusion...
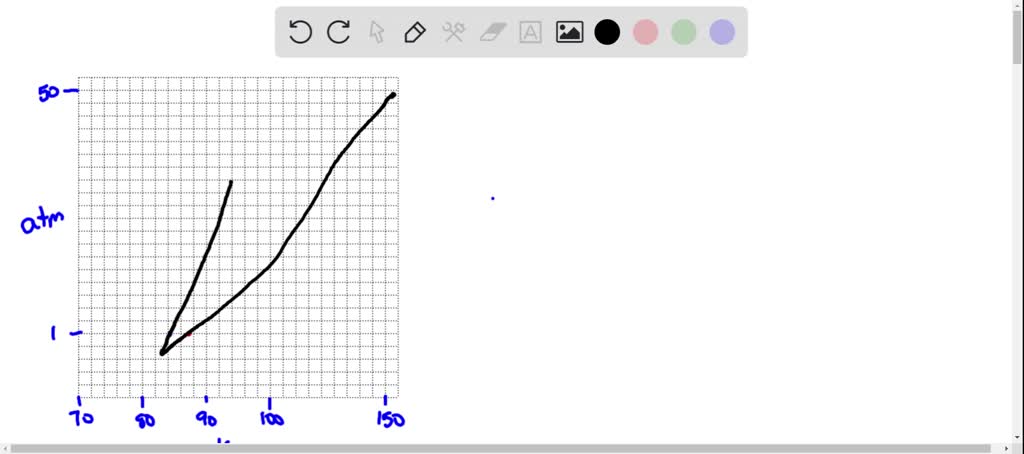
argon has a normal boiling point of 872 k and a melting point at 1 atm of 841 k its critical temperature is 1508 k and its critical pressure is 483 atm it has a triple point at 837 k and 068 atm ske 3
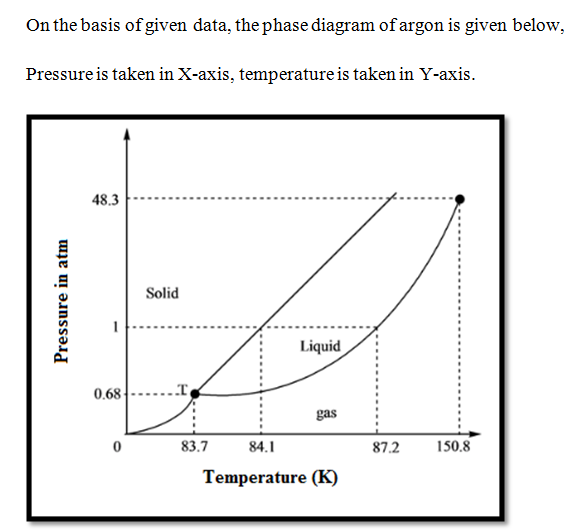













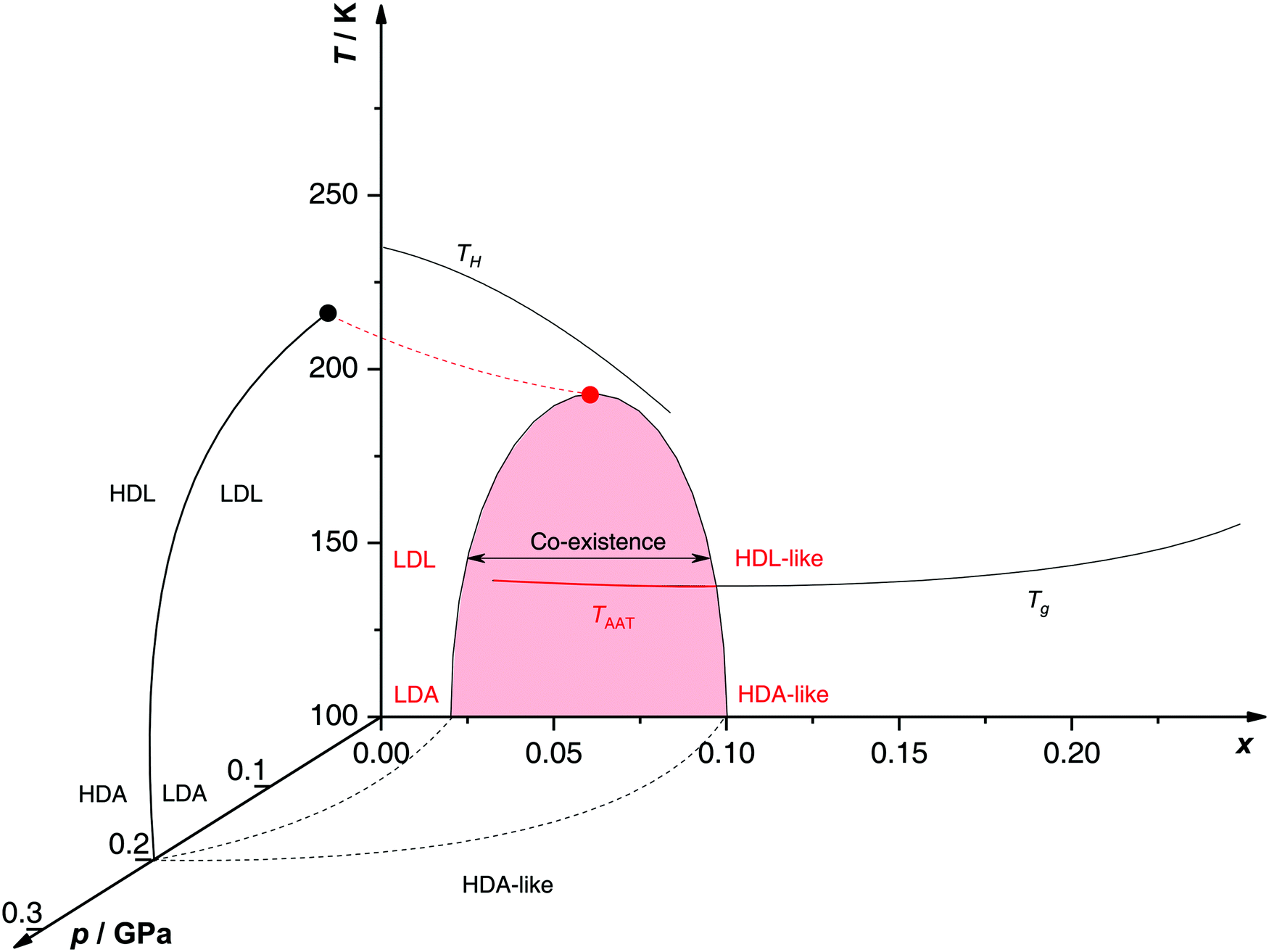


0 Response to "45 sketch the phase diagram to answer whether solid argon or liquid argon has the greater density?"
Post a Comment